| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor gamma |
|---|
| Ligand | BDBM50610147 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2280259 |
|---|
| EC50 | 3.4±n/a nM |
|---|
| Citation |  Orsi, DL; Ferrara, SJ; Siegel, S; Friberg, A; Bouchť, L; Pook, E; Lienau, P; Bluck, JP; Lemke, CT; Akcay, G; Stellfeld, T; Meyer, H; PŁtter, V; Holton, SJ; Korr, D; Jerchel-Furau, I; Pantelidou, C; Strathdee, CA; Meyerson, M; Eis, K; Goldstein, JT Discovery and characterization of orally bioavailable 4-chloro-6-fluoroisophthalamides as covalent PPARG inverse-agonists. Bioorg Med Chem78:0 (2023) [PubMed] Orsi, DL; Ferrara, SJ; Siegel, S; Friberg, A; Bouchť, L; Pook, E; Lienau, P; Bluck, JP; Lemke, CT; Akcay, G; Stellfeld, T; Meyer, H; PŁtter, V; Holton, SJ; Korr, D; Jerchel-Furau, I; Pantelidou, C; Strathdee, CA; Meyerson, M; Eis, K; Goldstein, JT Discovery and characterization of orally bioavailable 4-chloro-6-fluoroisophthalamides as covalent PPARG inverse-agonists. Bioorg Med Chem78:0 (2023) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor gamma |
|---|
| Name: | Peroxisome proliferator-activated receptor gamma |
|---|
| Synonyms: | NR1C3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG | PPARG_HUMAN | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma (PPAR gamma) | Peroxisome proliferator-activated receptor gamma (PPARG) | Peroxisome proliferator-activated receptor gamma (PPARő≥) | Peroxisome proliferator-activated receptor gamma/Nuclear receptor corepressor 2 | peroxisome proliferator-activated receptor gamma isoform 2 |
|---|
| Type: | Nuclear Receptor |
|---|
| Mol. Mass.: | 57613.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P37231 |
|---|
| Residue: | 505 |
|---|
| Sequence: | MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSF
DIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKT
QLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNC
RIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLR
ALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQE
QSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLAS
LMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVII
LSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQL
LQVIKKTETDMSLHPLLQEIYKDLY
|
|
|
|---|
| BDBM50610147 |
|---|
| n/a |
|---|
| Name | BDBM50610147 |
|---|
| Synonyms: | CHEMBL5289955 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H16Cl2N2O2 |
|---|
| Mol. Mass. | 399.27 |
|---|
| SMILES | Clc1cc(cc(C(=O)Nc2ccccc2)c1Cl)C(=O)NCc1ccccc1 |
|---|
| Structure | 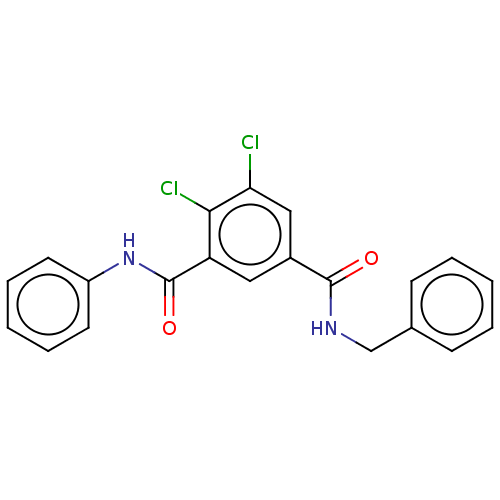
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Orsi, DL; Ferrara, SJ; Siegel, S; Friberg, A; Bouchť, L; Pook, E; Lienau, P; Bluck, JP; Lemke, CT; Akcay, G; Stellfeld, T; Meyer, H; PŁtter, V; Holton, SJ; Korr, D; Jerchel-Furau, I; Pantelidou, C; Strathdee, CA; Meyerson, M; Eis, K; Goldstein, JT Discovery and characterization of orally bioavailable 4-chloro-6-fluoroisophthalamides as covalent PPARG inverse-agonists. Bioorg Med Chem78:0 (2023) [PubMed]
Orsi, DL; Ferrara, SJ; Siegel, S; Friberg, A; Bouchť, L; Pook, E; Lienau, P; Bluck, JP; Lemke, CT; Akcay, G; Stellfeld, T; Meyer, H; PŁtter, V; Holton, SJ; Korr, D; Jerchel-Furau, I; Pantelidou, C; Strathdee, CA; Meyerson, M; Eis, K; Goldstein, JT Discovery and characterization of orally bioavailable 4-chloro-6-fluoroisophthalamides as covalent PPARG inverse-agonists. Bioorg Med Chem78:0 (2023) [PubMed]