| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein phosphatase F |
|---|
| Ligand | BDBM50118779 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2286043 |
|---|
| Ki | 2000000±n/a nM |
|---|
| Citation |  Kousaxidis, A; Petrou, A; Lavrentaki, V; Fesatidou, M; Nicolaou, I; Geronikaki, A Aldose reductase and protein tyrosine phosphatase 1B inhibitors as a promising therapeutic approach for diabetes mellitus. Eur J Med Chem207:0 (2020) [PubMed] Kousaxidis, A; Petrou, A; Lavrentaki, V; Fesatidou, M; Nicolaou, I; Geronikaki, A Aldose reductase and protein tyrosine phosphatase 1B inhibitors as a promising therapeutic approach for diabetes mellitus. Eur J Med Chem207:0 (2020) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein phosphatase F |
|---|
| Name: | Receptor-type tyrosine-protein phosphatase F |
|---|
| Synonyms: | LAR | Leukocyte common antigen related | Leukocyte common antigen related (LAR) | PTPRF | PTPRF_HUMAN | Receptor-type tyrosine-protein phosphatase F | Receptor-type tyrosine-protein phosphatase F (LAR) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 212869.85 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10586 |
|---|
| Residue: | 1907 |
|---|
| Sequence: | MAPEPAPGRTMVPLVPALVMLGLVAGAHGDSKPVFIKVPEDQTGLSGGVASFVCQATGEP
KPRITWMKKGKKVSSQRFEVIEFDDGAGSVLRIQPLRVQRDEAIYECTATNSLGEINTSA
KLSVLEEEQLPPGFPSIDMGPQLKVVEKARTATMLCAAGGNPDPEISWFKDFLPVDPATS
NGRIKQLRSGALQIESSEESDQGKYECVATNSAGTRYSAPANLYVRVRRVAPRFSIPPSS
QEVMPGGSVNLTCVAVGAPMPYVKWMMGAEELTKEDEMPVGRNVLELSNVVRSANYTCVA
ISSLGMIEATAQVTVKALPKPPIDLVVTETTATSVTLTWDSGNSEPVTYYGIQYRAAGTE
GPFQEVDGVATTRYSIGGLSPFSEYAFRVLAVNSIGRGPPSEAVRARTGEQAPSSPPRRV
QARMLSASTMLVQWEPPEEPNGLVRGYRVYYTPDSRRPPNAWHKHNTDAGLLTTVGSLLP
GITYSLRVLAFTAVGDGPPSPTIQVKTQQGVPAQPADFQAEVESDTRIQLSWLLPPQERI
IMYELVYWAAEDEDQQHKVTFDPTSSYTLEDLKPDTLYRFQLAARSDMGVGVFTPTIEAR
TAQSTPSAPPQKVMCVSMGSTTVRVSWVPPPADSRNGVITQYSVAYEAVDGEDRGRHVVD
GISREHSSWDLVGLEKWTEYRVWVRAHTDVGPGPESSPVLVRTDEDVPSGPPRKVEVEPL
NSTAVHVYWKLPVPSKQHGQIRGYQVTYVRLENGEPRGLPIIQDVMLAEAQWRPEESEDY
ETTISGLTPETTYSVTVAAYTTKGDGARSKPKIVTTTGAVPGRPTMMISTTAMNTALLQW
HPPKELPGELLGYRLQYCRADEARPNTIDFGKDDQHFTVTGLHKGTTYIFRLAAKNRAGL
GEEFEKEIRTPEDLPSGFPQNLHVTGLTTSTTELAWDPPVLAERNGRIISYTVVFRDINS
QQELQNITTDTRFTLTGLKPDTTYDIKVRAWTSKGSGPLSPSIQSRTMPVEQVFAKNFRV
AAAMKTSVLLSWEVPDSYKSAVPFKILYNGQSVEVDGHSMRKLIADLQPNTEYSFVLMNR
GSSAGGLQHLVSIRTAPDLLPHKPLPASAYIEDGRFDLSMPHVQDPSLVRWFYIVVVPID
RVGGSMLTPRWSTPEELELDELLEAIEQGGEEQRRRRRQAERLKPYVAAQLDVLPETFTL
GDKKNYRGFYNRPLSPDLSYQCFVLASLKEPMDQKRYASSPYSDEIVVQVTPAQQQEEPE
MLWVTGPVLAVILIILIVIAILLFKRKRTHSPSSKDEQSIGLKDSLLAHSSDPVEMRRLN
YQTPGMRDHPPIPITDLADNIERLKANDGLKFSQEYESIDPGQQFTWENSNLEVNKPKNR
YANVIAYDHSRVILTSIDGVPGSDYINANYIDGYRKQNAYIATQGPLPETMGDFWRMVWE
QRTATVVMMTRLEEKSRVKCDQYWPARGTETCGLIQVTLLDTVELATYTVRTFALHKSGS
SEKRELRQFQFMAWPDHGVPEYPTPILAFLRRVKACNPLDAGPMVVHCSAGVGRTGCFIV
IDAMLERMKHEKTVDIYGHVTCMRSQRNYMVQTEDQYVFIHEALLEAATCGHTEVPARNL
YAHIQKLGQVPPGESVTAMELEFKLLASSKAHTSRFISANLPCNKFKNRLVNIMPYELTR
VCLQPIRGVEGSDYINASFLDGYRQQKAYIATQGPLAESTEDFWRMLWEHNSTIIVMLTK
LREMGREKCHQYWPAERSARYQYFVVDPMAEYNMPQYILREFKVTDARDGQSRTIRQFQF
TDWPEQGVPKTGEGFIDFIGQVHKTKEQFGQDGPITVHCSAGVGRTGVFITLSIVLERMR
YEGVVDMFQTVKTLRTQRPAMVQTEDQYQLCYRAALEYLGSFDHYAT
|
|
|
|---|
| BDBM50118779 |
|---|
| n/a |
|---|
| Name | BDBM50118779 |
|---|
| Synonyms: | 3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(carboxyformamido)thiophene-2-carboxylic acid | CHEMBL336879 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C7H5NO5S |
|---|
| Mol. Mass. | 215.183 |
|---|
| SMILES | OC(=O)C(=O)Nc1ccsc1C(O)=O |
|---|
| Structure | 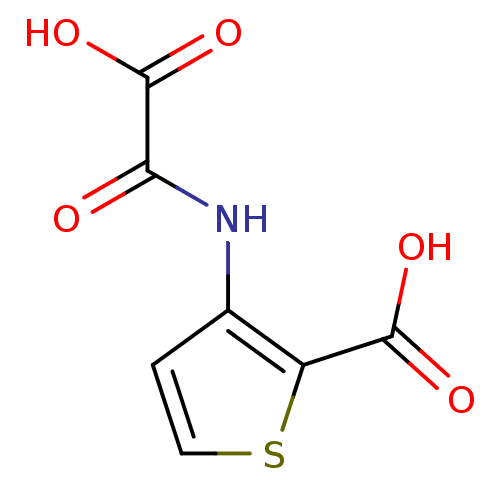
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kousaxidis, A; Petrou, A; Lavrentaki, V; Fesatidou, M; Nicolaou, I; Geronikaki, A Aldose reductase and protein tyrosine phosphatase 1B inhibitors as a promising therapeutic approach for diabetes mellitus. Eur J Med Chem207:0 (2020) [PubMed]
Kousaxidis, A; Petrou, A; Lavrentaki, V; Fesatidou, M; Nicolaou, I; Geronikaki, A Aldose reductase and protein tyrosine phosphatase 1B inhibitors as a promising therapeutic approach for diabetes mellitus. Eur J Med Chem207:0 (2020) [PubMed]