

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Alpha-1D adrenergic receptor | ||
| Ligand | BDBM50211327 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_444278 (CHEMBL894518) | ||
| Ki | 1.5±n/a nM | ||
| Citation |  Chiu, G; Li, S; Connolly, PJ; Pulito, V; Liu, J; Middleton, SA (Arylpiperazinyl)cyclohexylsufonamides: discovery of alpha(1a/1d)-selective adrenergic receptor antagonists for the treatment of Benign Prostatic Hyperplasia/Lower Urinary Tract Symptoms (BPH/LUTS). Bioorg Med Chem Lett17:3292-7 (2007) [PubMed] Article Chiu, G; Li, S; Connolly, PJ; Pulito, V; Liu, J; Middleton, SA (Arylpiperazinyl)cyclohexylsufonamides: discovery of alpha(1a/1d)-selective adrenergic receptor antagonists for the treatment of Benign Prostatic Hyperplasia/Lower Urinary Tract Symptoms (BPH/LUTS). Bioorg Med Chem Lett17:3292-7 (2007) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Alpha-1D adrenergic receptor | |||
| Name: | Alpha-1D adrenergic receptor | ||
| Synonyms: | ADA1D_HUMAN | ADRA1A | ADRA1D | Adrenergic receptor | Adrenergic receptor alpha | Alpha 1D-adrenoceptor | Alpha 1D-adrenoreceptor | Alpha adrenergic receptor (1a and 1d) | Alpha-1D adrenoceptor | Alpha-adrenergic receptor 1a | adrenergic Alpha1D | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 60485.82 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | adrenergic Alpha1D ADRA1D HUMAN::P25100 | ||
| Residue: | 572 | ||
| Sequence: |
| ||
| BDBM50211327 | |||
| n/a | |||
| Name | BDBM50211327 | ||
| Synonyms: | 4-chloro-2-fluoro-N-((1s,4s)-4-(4-(2-isopropoxyphenyl)piperazin-1-yl)cyclohexyl)benzenesulfonamide | CHEMBL233410 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C25H33ClFN3O3S | ||
| Mol. Mass. | 510.064 | ||
| SMILES | CC(C)Oc1ccccc1N1CCN(CC1)[C@@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(Cl)cc1F |wU:16.17,19.24,(-9.41,-5.91,;-10.19,-7.23,;-11.73,-7.21,;-9.44,-8.57,;-10.23,-9.9,;-11.76,-9.88,;-12.57,-11.21,;-11.8,-12.55,;-10.25,-12.57,;-9.48,-11.24,;-7.95,-11.26,;-7.18,-12.6,;-5.64,-12.62,;-4.87,-11.3,;-5.61,-9.96,;-7.15,-9.94,;-3.33,-11.32,;-2.58,-12.66,;-1.04,-12.69,;-.26,-11.36,;-1,-10.02,;-2.55,-9.99,;1.3,-11.4,;2.08,-10.07,;.74,-9.29,;3.4,-10.83,;2.83,-8.72,;2.06,-7.41,;2.81,-6.07,;4.36,-6.05,;5.12,-4.71,;5.14,-7.38,;4.38,-8.72,;5.16,-10.05,)| | ||
| Structure | 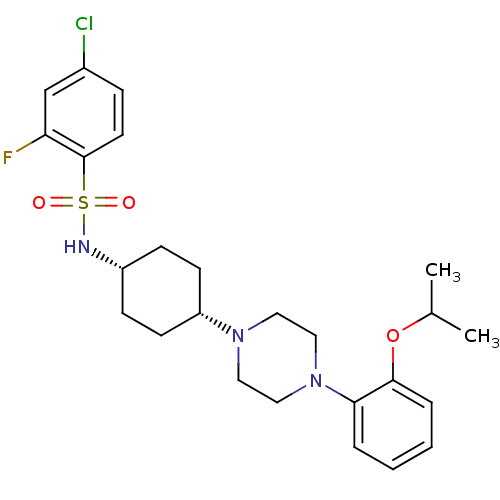
| ||