| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Renin |
|---|
| Ligand | BDBM50286817 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_195958 |
|---|
| IC50 | 3000±n/a nM |
|---|
| Citation |  Dee, MF; Rosati, RL Synthesis of α-hydroxy statine through a facially selective osmylation of a chiral α-amido crotonate Bioorg Med Chem Lett5:949-952 (1995) Article Dee, MF; Rosati, RL Synthesis of α-hydroxy statine through a facially selective osmylation of a chiral α-amido crotonate Bioorg Med Chem Lett5:949-952 (1995) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Renin |
|---|
| Name: | Renin |
|---|
| Synonyms: | Angiotensinogenase | REN | RENI_HUMAN |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 45058.99 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 406 |
|---|
| Sequence: | MDGWRRMPRWGLLLLLWGSCTFGLPTDTTTFKRIFLKRMPSIRESLKERGVDMARLGPEW
SQPMKRLTLGNTTSSVILTNYMDTQYYGEIGIGTPPQTFKVVFDTGSSNVWVPSSKCSRL
YTACVYHKLFDASDSSSYKHNGTELTLRYSTGTVSGFLSQDIITVGGITVTQMFGEVTEM
PALPFMLAEFDGVVGMGFIEQAIGRVTPIFDNIISQGVLKEDVFSFYYNRDSENSQSLGG
QIVLGGSDPQHYEGNFHYINLIKTGVWQIQMKGVSVGSSTLLCEDGCLALVDTGASYISG
STSSIEKLMEALGAKKRLFDYVVKCNEGPTLPDISFHLGGKEYTLTSADYVFQESYSSKK
LCTLAIHAMDIPPPTGPTWALGATFIRKFYTEFDRRNNRIGFALAR
|
|
|
|---|
| BDBM50286817 |
|---|
| n/a |
|---|
| Name | BDBM50286817 |
|---|
| Synonyms: | 4-{(2S,3R,4S)-4-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-phenyl-propionylamino)-3-(3H-imidazol-4-yl)-propionylamino]-2,3-dihydroxy-6-methyl-heptanoylamino}-butyric acid | CHEMBL172585 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C32H48N6O9 |
|---|
| Mol. Mass. | 660.7583 |
|---|
| SMILES | CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)[C@H](O)C(=O)NCCCC(O)=O |
|---|
| Structure | 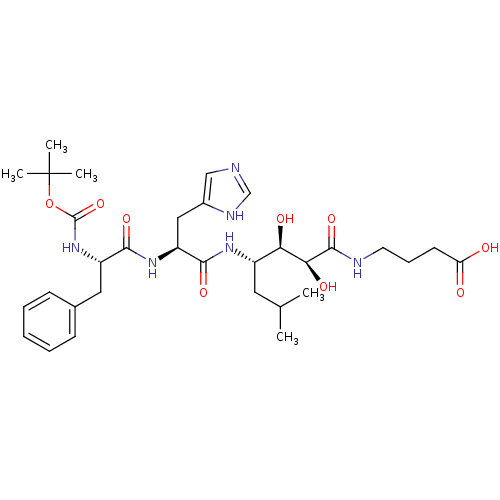
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Dee, MF; Rosati, RL Synthesis of α-hydroxy statine through a facially selective osmylation of a chiral α-amido crotonate Bioorg Med Chem Lett5:949-952 (1995) Article
Dee, MF; Rosati, RL Synthesis of α-hydroxy statine through a facially selective osmylation of a chiral α-amido crotonate Bioorg Med Chem Lett5:949-952 (1995) Article