| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Thioredoxin glutathione reductase |
|---|
| Ligand | BDBM50300761 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_589927 (CHEMBL1048278) |
|---|
| IC50 | 400±n/a nM |
|---|
| Citation |  Rai, G; Sayed, AA; Lea, WA; Luecke, HF; Chakrapani, H; Prast-Nielsen, S; Jadhav, A; Leister, W; Shen, M; Inglese, J; Austin, CP; Keefer, L; Arnér, ES; Simeonov, A; Maloney, DJ; Williams, DL; Thomas, CJ Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem52:6474-83 (2009) [PubMed] Article Rai, G; Sayed, AA; Lea, WA; Luecke, HF; Chakrapani, H; Prast-Nielsen, S; Jadhav, A; Leister, W; Shen, M; Inglese, J; Austin, CP; Keefer, L; Arnér, ES; Simeonov, A; Maloney, DJ; Williams, DL; Thomas, CJ Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem52:6474-83 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Thioredoxin glutathione reductase |
|---|
| Name: | Thioredoxin glutathione reductase |
|---|
| Synonyms: | n/a |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 64881.48 |
|---|
| Organism: | Schistosoma mansoni |
|---|
| Description: | ChEMBL_1455608 |
|---|
| Residue: | 597 |
|---|
| Sequence: | MPPADGTSQWLRKTVDSAAVILFSKTTCPYCKKVKDVLAEAKIKHATIELDQLSNGSAIQ
KCLASFSKIETVPQMFVRGKFIGDSQTVLKYYSNDELAGIVNESKYDYDLIVIGGGSGGL
AAGKEAAKYGAKTAVLDYVEPTPIGTTWGLGGTCVNVGCIPKKLMHQAGLLSHALEDAEH
FGWSLDRSKISHNWSTMVEGVQSHIGSLNWGYKVALRDNQVTYLNAKGRLISPHEVQITD
KNQKVSTITGNKIILATGERPKYPEIPGAVEYGITSDDLFSLPYFPGKTLVIGASYVALE
CAGFLASLGGDVTVMVRSILLRGFDQQMAEKVGDYMENHGVKFAKLCVPDEIKQLKVVDT
ENNKPGLLLVKGHYTDGKKFEEEFETVIFAVGREPQLSKVLCETVGVKLDKNGRVVCTDD
EQTTVSNVYAIGDINAGKPQLTPVAIQAGRYLARRLFAGATELTDYSNVATTVFTPLEYG
ACGLSEEDAIEKYGDKDIEVYHSNFKPLEWTVAHREDNVCYMKLVCRKSDNMRVLGLHVL
GPNAGEITQGYAVAIKMGATKADFDRTIGIHPTCSETFTTLHVTKKSGVSPIVSGCG
|
|
|
|---|
| BDBM50300761 |
|---|
| n/a |
|---|
| Name | BDBM50300761 |
|---|
| Synonyms: | 4,4'-(thiophene-2,5-diyl)bis(3-cyano-1,2,5-oxadiazole 2-oxide) | CHEMBL569420 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H2N6O4S |
|---|
| Mol. Mass. | 302.226 |
|---|
| SMILES | [O-][n+]1onc(-c2ccc(s2)-c2no[n+]([O-])c2C#N)c1C#N |
|---|
| Structure | 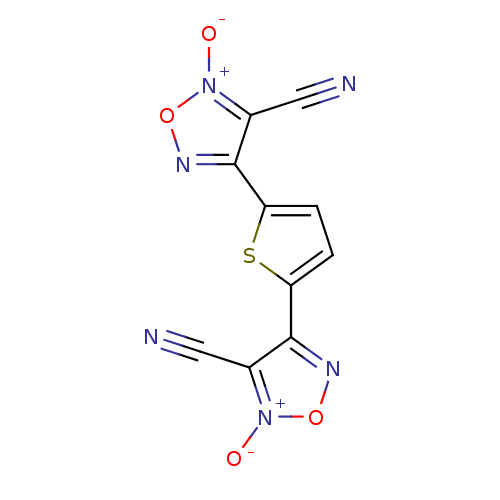
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Rai, G; Sayed, AA; Lea, WA; Luecke, HF; Chakrapani, H; Prast-Nielsen, S; Jadhav, A; Leister, W; Shen, M; Inglese, J; Austin, CP; Keefer, L; Arnér, ES; Simeonov, A; Maloney, DJ; Williams, DL; Thomas, CJ Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem52:6474-83 (2009) [PubMed] Article
Rai, G; Sayed, AA; Lea, WA; Luecke, HF; Chakrapani, H; Prast-Nielsen, S; Jadhav, A; Leister, W; Shen, M; Inglese, J; Austin, CP; Keefer, L; Arnér, ES; Simeonov, A; Maloney, DJ; Williams, DL; Thomas, CJ Structure mechanism insights and the role of nitric oxide donation guide the development of oxadiazole-2-oxides as therapeutic agents against schistosomiasis. J Med Chem52:6474-83 (2009) [PubMed] Article