

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50068039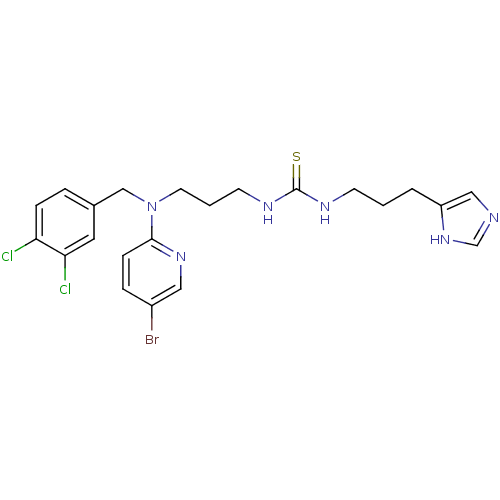 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50496732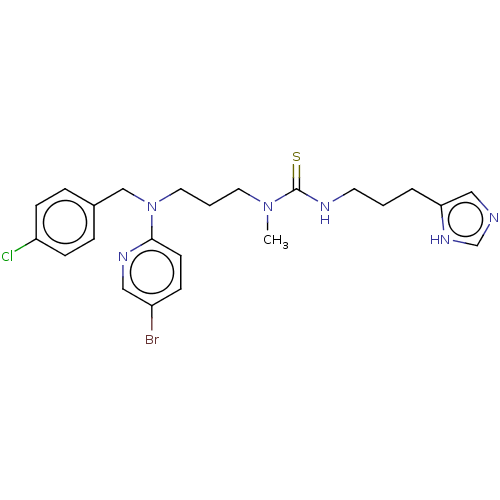 (CHEMBL1591268) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50496735 (CHEMBL1355048) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50496733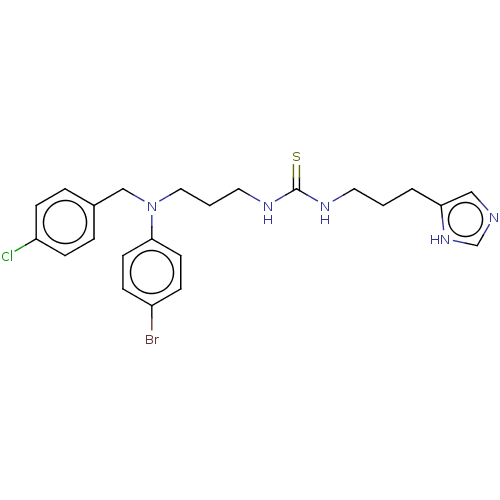 (CHEMBL1591395) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50496734 (CHEMBL1590268) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18428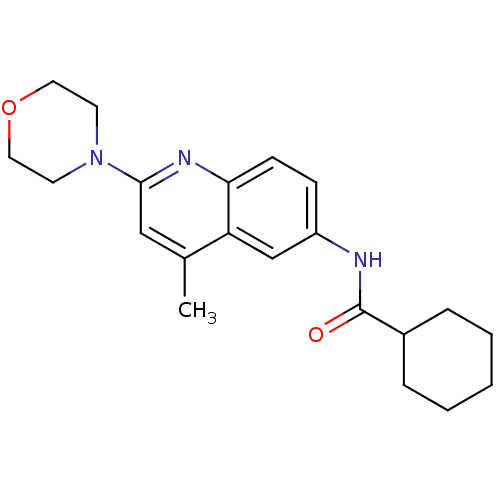 (Aminoquinoline compound, 1 | N-[4-methyl-2-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801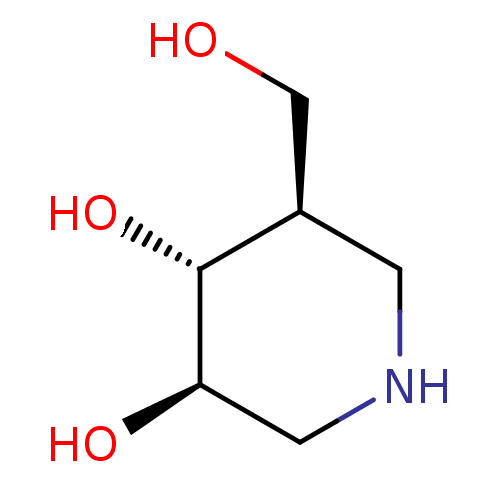 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM228659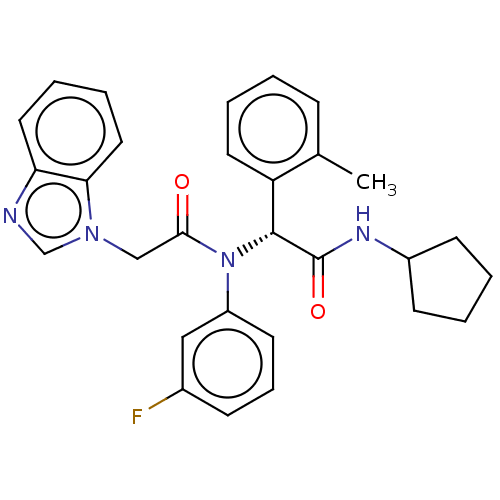 ((+)-2-(2-(1H-Benzo[d]imidazol-1-yl)-N-(3-fluorophe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The activity of IDH1 R132H and IDH1 R132C was measured in 384-well plates by coupling NADPH consumption to a diaphorase/resazurin-based detection sys... | J Biol Chem 289: 13717-25 (2014) Article DOI: 10.1074/jbc.M113.511030 BindingDB Entry DOI: 10.7270/Q2513X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM35361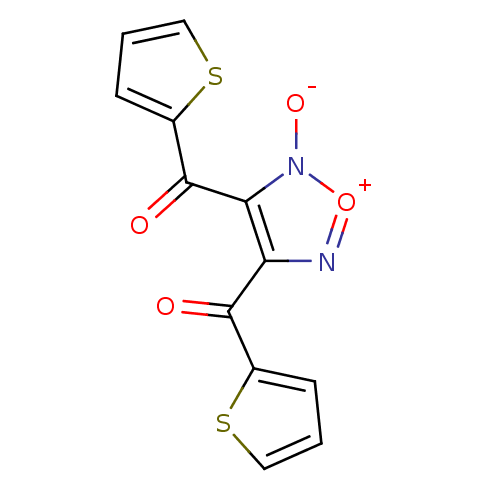 ((5-oxidanidyl-4-thiophen-2-ylcarbonyl-1,2,5-oxadia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM228658 (rac-ML309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The activity of IDH1 R132H and IDH1 R132C was measured in 384-well plates by coupling NADPH consumption to a diaphorase/resazurin-based detection sys... | J Biol Chem 289: 13717-25 (2014) Article DOI: 10.1074/jbc.M113.511030 BindingDB Entry DOI: 10.7270/Q2513X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300734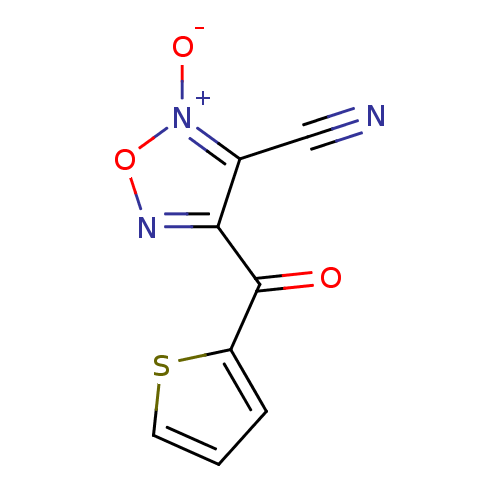 (4-thienoyl-3-cyanofuroxan | CHEMBL576265) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM228659 ((+)-2-(2-(1H-Benzo[d]imidazol-1-yl)-N-(3-fluorophe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The activity of IDH1 R132H and IDH1 R132C was measured in 384-well plates by coupling NADPH consumption to a diaphorase/resazurin-based detection sys... | J Biol Chem 289: 13717-25 (2014) Article DOI: 10.1074/jbc.M113.511030 BindingDB Entry DOI: 10.7270/Q2513X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM228658 (rac-ML309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The activity of IDH1 R132H and IDH1 R132C was measured in 384-well plates by coupling NADPH consumption to a diaphorase/resazurin-based detection sys... | J Biol Chem 289: 13717-25 (2014) Article DOI: 10.1074/jbc.M113.511030 BindingDB Entry DOI: 10.7270/Q2513X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18438 (4-benzenesulfonamido-N-(5-ethyl-1,3,4-thiadiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300756 (3-formyl-4-phenyl-1,2,5-oxadiazole 2-oxide | CHEMB...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50396168 (CHEMBL1361379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase in human spleen homogenate using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to subs... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300760 (4,4'-(thiophene-2,4-diyl)bis(3-cyano-1,2,5-oxadiaz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase in human spleen homogenate using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to subs... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18447 (2-({4-[(5-chloro-2-methoxyphenyl)amino]-6-(pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300761 (4,4'-(thiophene-2,5-diyl)bis(3-cyano-1,2,5-oxadiaz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300762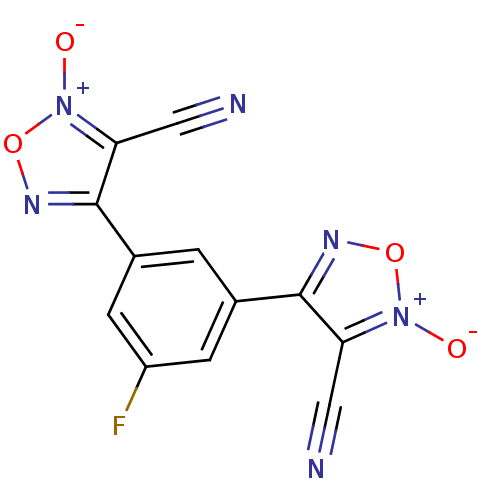 (4,4'-(5-fluoro-1,3-phenylene)bis(3-cyano-1,2,5-oxa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300755 (3-carboxy-4-phenyl-1,2,5-oxadiazole 2-oxide | CHEM...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398032 (CHEMBL1603014) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398032 (CHEMBL1603014) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300763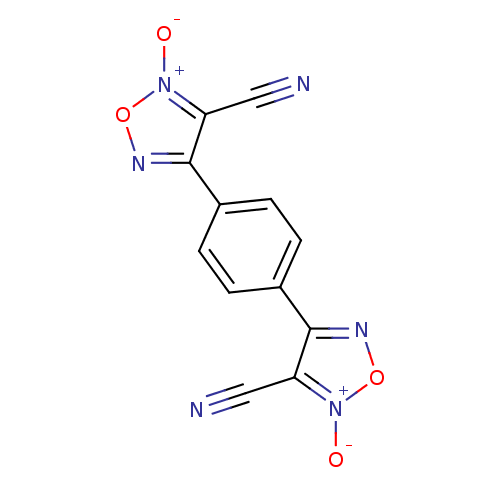 (4,4'-(1,4-phenylene)bis(3-cyano-1,2,5-oxadiazole 2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398022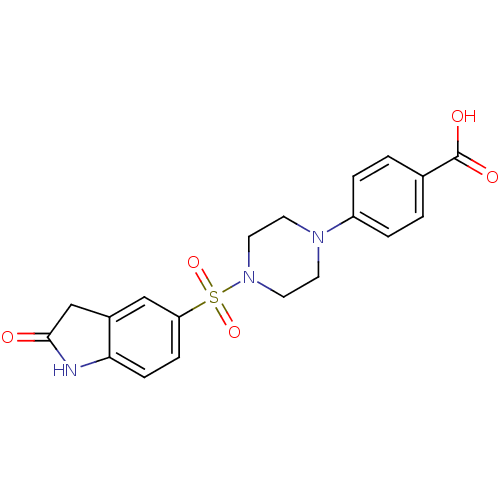 (CHEMBL2181097) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398022 (CHEMBL2181097) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398020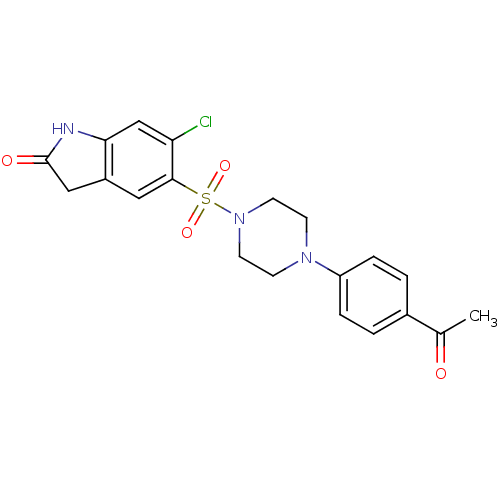 (CHEMBL2181103) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398020 (CHEMBL2181103) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398029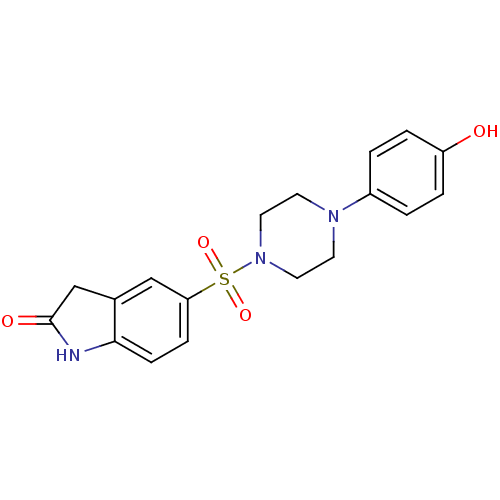 (CHEMBL2180814) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18428 (Aminoquinoline compound, 1 | N-[4-methyl-2-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase in human spleen homogenate using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to subs... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300753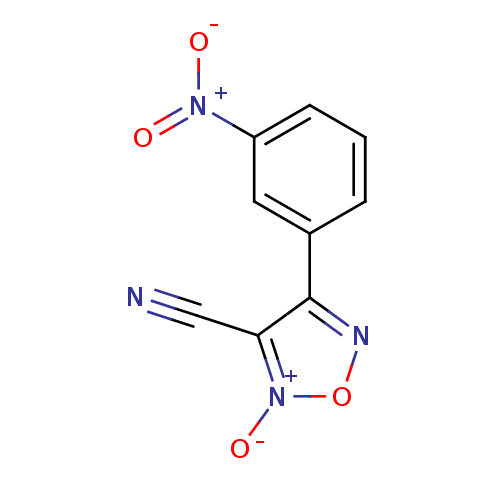 (3-cyano-4-(3-nitrophenyl)-1,2,5-oxadiazole 2-oxide...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398029 (CHEMBL2180814) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300752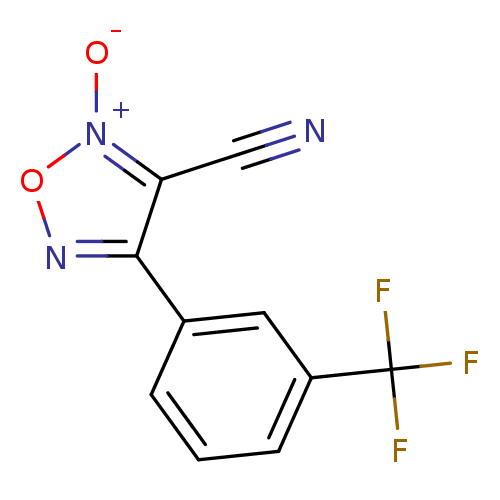 (3-cyano-4-(3-(trifluoromethyl)phenyl)-1,2,5-oxadia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300750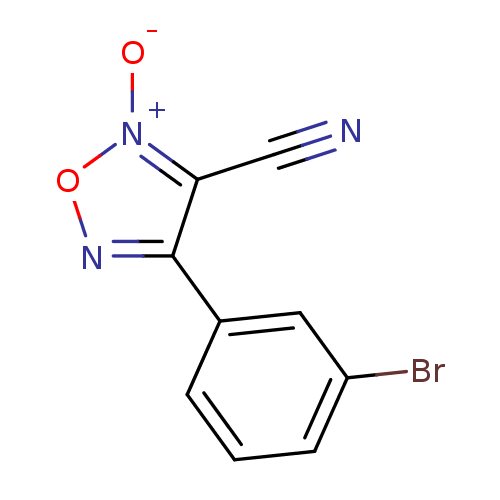 (4-(3-bromophenyl)-3-cyano-1,2,5-oxadiazole 2-oxide...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300736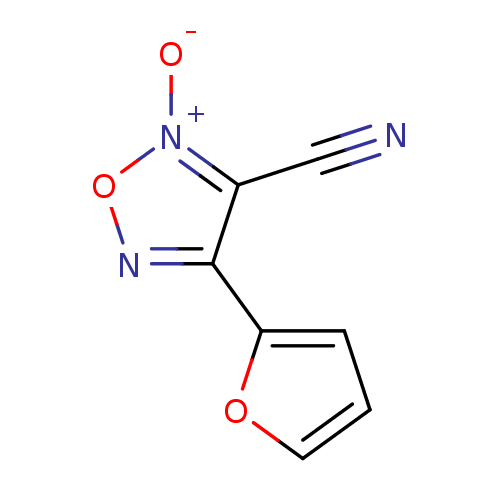 (3-cyano-4-(furan-2-yl)-1,2,5-oxadiazole 2-oxide | ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300751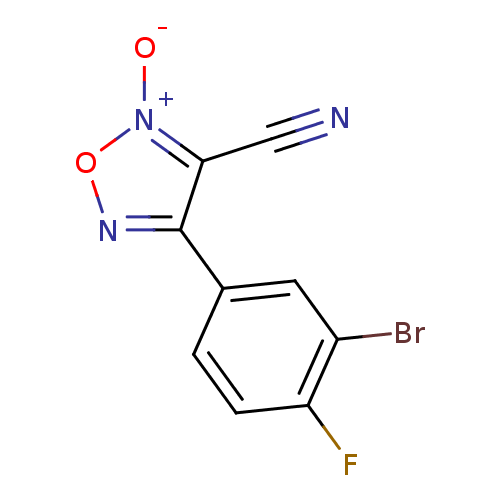 (4-(3-bromo-4-fluorophenyl)-3-cyano-1,2,5-oxadiazol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eyes absent homolog 2 [253-538] (Homo sapiens (Human)) | BDBM231626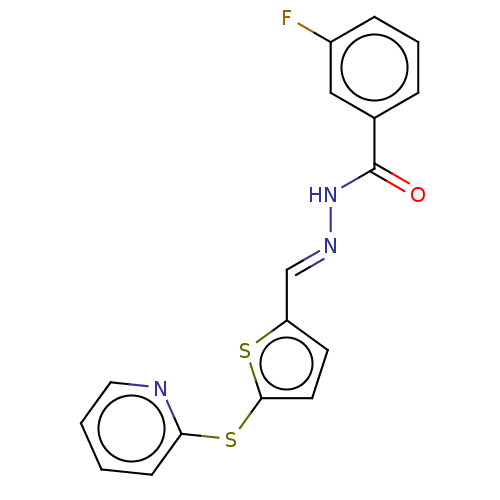 (NCGC00249327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 6.5 | 25 |
University of Colorado School of Medicine | Assay Description Eya activity was measured in 50-μl reactions using black, 96-well, half-volume microtiter plates (Greiner Bio-one) with the substrate OMFP (3-O-... | J Biol Chem 289: 16349-61 (2014) Article DOI: 10.1074/jbc.M114.566729 BindingDB Entry DOI: 10.7270/Q2CC0ZJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398028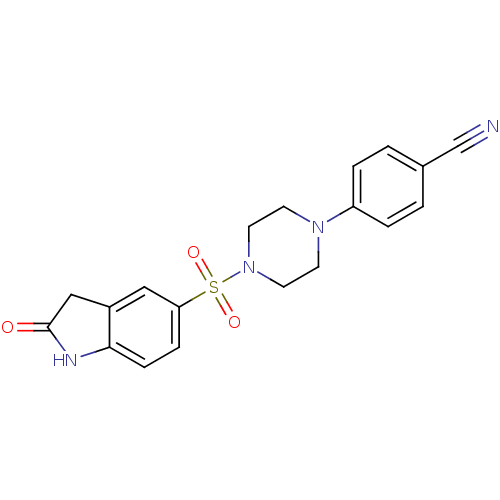 (CHEMBL2180816) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18438 (4-benzenesulfonamido-N-(5-ethyl-1,3,4-thiadiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase in human spleen homogenate using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to subs... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398027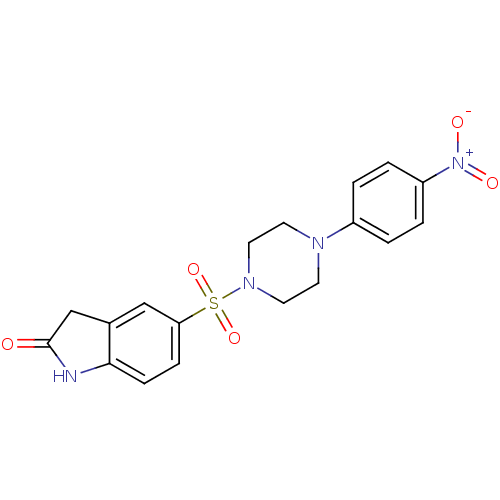 (CHEMBL2181089) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398028 (CHEMBL2180816) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300735 (3-cyano-4-(thiophen-2-yl)-1,2,5-oxadiazole 2-oxide...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300748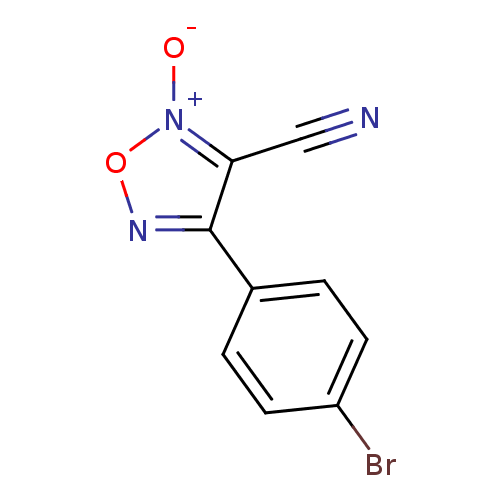 (4-(4-bromophenyl)-3-cyano-1,2,5-oxadiazole 2-oxide...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300749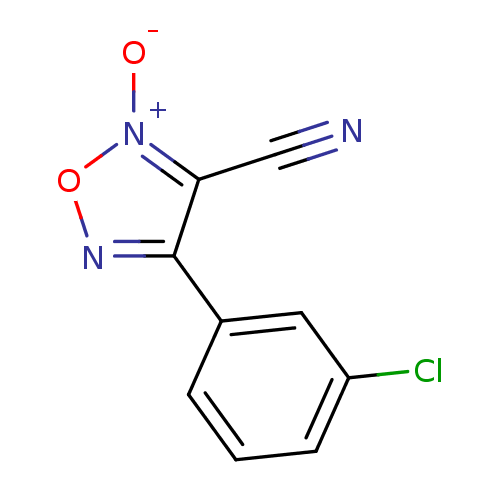 (4-(3-chlorophenyl)-3-cyano-1,2,5-oxadiazole 2-oxid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300759 (4,4'-(1,3-phenylene)bis(3-cyano-1,2,5-oxadiazole 2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398027 (CHEMBL2181089) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398024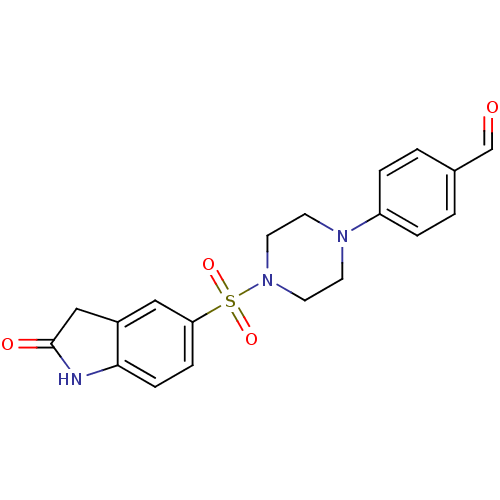 (CHEMBL2181095) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50300747 (4-(4-chlorophenyl)-3-cyano-1,2,5-oxadiazole 2-oxid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni TGR | J Med Chem 52: 6474-83 (2009) Article DOI: 10.1021/jm901021k BindingDB Entry DOI: 10.7270/Q2TH8MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 135 total ) | Next | Last >> |