| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cathepsin D |
|---|
| Ligand | BDBM50316599 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_631746 (CHEMBL1114767) |
|---|
| IC50 | 50±n/a nM |
|---|
| Citation |  Gupta, D; Yedidi, RS; Varghese, S; Kovari, LC; Woster, PM Mechanism-based inhibitors of the aspartyl protease plasmepsin II as potential antimalarial agents. J Med Chem53:4234-47 (2010) [PubMed] Article Gupta, D; Yedidi, RS; Varghese, S; Kovari, LC; Woster, PM Mechanism-based inhibitors of the aspartyl protease plasmepsin II as potential antimalarial agents. J Med Chem53:4234-47 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cathepsin D |
|---|
| Name: | Cathepsin D |
|---|
| Synonyms: | CATD_HUMAN | CPSD | CTSD | Cathepsin D [Precursor] | Cathepsin D heavy chain | Cathepsin D light chain | Cathepsin D precursor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 44551.72 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human proCathepsin D (SwissProt accession number P07339) was expressed in Sf9 cells, purified, and autoactivated. |
|---|
| Residue: | 412 |
|---|
| Sequence: | MQPSSLLPLALCLLAAPASALVRIPLHKFTSIRRTMSEVGGSVEDLIAKGPVSKYSQAVP
AVTEGPIPEVLKNYMDAQYYGEIGIGTPPQCFTVVFDTGSSNLWVPSIHCKLLDIACWIH
HKYNSDKSSTYVKNGTSFDIHYGSGSLSGYLSQDTVSVPCQSASSASALGGVKVERQVFG
EATKQPGITFIAAKFDGILGMAYPRISVNNVLPVFDNLMQQKLVDQNIFSFYLSRDPDAQ
PGGELMLGGTDSKYYKGSLSYLNVTRKAYWQVHLDQVEVASGLTLCKEGCEAIVDTGTSL
MVGPVDEVRELQKAIGAVPLIQGEYMIPCEKVSTLPAITLKLGGKGYKLSPEDYTLKVSQ
AGKTLCLSGFMGMDIPPPSGPLWILGDVFIGRYYTVFDRDNNRVGFAEAARL
|
|
|
|---|
| BDBM50316599 |
|---|
| n/a |
|---|
| Name | BDBM50316599 |
|---|
| Synonyms: | CHEMBL1098669 | N-((S)-1-((2S,3S)-5-((S)-1-((S)-1-amino-4-methyl-1-oxopentan-2-ylamino)-3-cyano-1-oxopropan-2-ylamino)-3-hydroxy-5-oxo-1-phenylpentan-2-ylamino)-3-methyl-1-oxobutan-2-yl)picolinamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C32H43N7O6 |
|---|
| Mol. Mass. | 621.7271 |
|---|
| SMILES | CC(C)C[C@H](NC(=O)[C@H](CC#N)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccccn1)C(C)C)C(N)=O |r| |
|---|
| Structure | 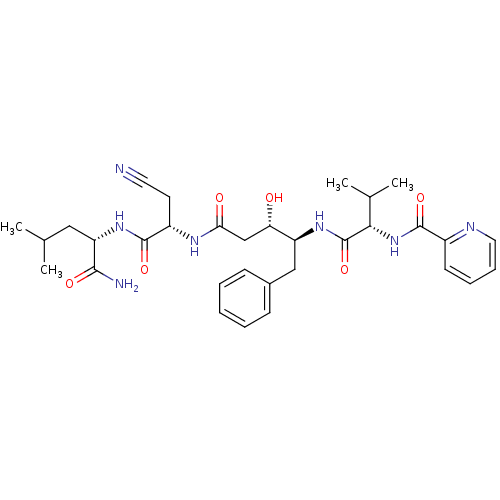
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gupta, D; Yedidi, RS; Varghese, S; Kovari, LC; Woster, PM Mechanism-based inhibitors of the aspartyl protease plasmepsin II as potential antimalarial agents. J Med Chem53:4234-47 (2010) [PubMed] Article
Gupta, D; Yedidi, RS; Varghese, S; Kovari, LC; Woster, PM Mechanism-based inhibitors of the aspartyl protease plasmepsin II as potential antimalarial agents. J Med Chem53:4234-47 (2010) [PubMed] Article