| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Polyunsaturated fatty acid lipoxygenase ALOX15 |
|---|
| Ligand | BDBM50206257 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_502461 (CHEMBL986634) |
|---|
| IC50 | 900±n/a nM |
|---|
| Citation |  Amagata, T; Whitman, S; Johnson, TA; Stessman, CC; Loo, CP; Lobkovsky, E; Clardy, J; Crews, P; Holman, TR Exploring sponge-derived terpenoids for their potency and selectivity against 12-human, 15-human, and 15-soybean lipoxygenases. J Nat Prod66:230-5 (2003) [PubMed] Article Amagata, T; Whitman, S; Johnson, TA; Stessman, CC; Loo, CP; Lobkovsky, E; Clardy, J; Crews, P; Holman, TR Exploring sponge-derived terpenoids for their potency and selectivity against 12-human, 15-human, and 15-soybean lipoxygenases. J Nat Prod66:230-5 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Polyunsaturated fatty acid lipoxygenase ALOX15 |
|---|
| Name: | Polyunsaturated fatty acid lipoxygenase ALOX15 |
|---|
| Synonyms: | 15-Lipoxygenase-1 (15-LOX-1) | ALOX15 | Arachidonate 15-lipoxygenase | Arachidonate 15-lipoxygenase-1 | LOG15 | LOX15_HUMAN | Reticulocyte 15-lipoxygenase-1 | Sphingosine 1-phosphate receptor 1 (S1P1) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 74804.05 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 662 |
|---|
| Sequence: | MGLYRIRVSTGASLYAGSNNQVQLWLVGQHGEAALGKRLWPARGKETELKVEVPEYLGPL
LFVKLRKRHLLKDDAWFCNWISVQGPGAGDEVRFPCYRWVEGNGVLSLPEGTGRTVGEDP
QGLFQKHREEELEERRKLYRWGNWKDGLILNMAGAKLYDLPVDERFLEDKRVDFEVSLAK
GLADLAIKDSLNVLTCWKDLDDFNRIFWCGQSKLAERVRDSWKEDALFGYQFLNGANPVV
LRRSAHLPARLVFPPGMEELQAQLEKELEGGTLFEADFSLLDGIKANVILCSQQHLAAPL
VMLKLQPDGKLLPMVIQLQLPRTGSPPPPLFLPTDPPMAWLLAKCWVRSSDFQLHELQSH
LLRGHLMAEVIVVATMRCLPSIHPIFKLIIPHLRYTLEINVRARTGLVSDMGIFDQIMST
GGGGHVQLLKQAGAFLTYSSFCPPDDLADRGLLGVKSSFYAQDALRLWEIIYRYVEGIVS
LHYKTDVAVKDDPELQTWCREITEIGLQGAQDRGFPVSLQARDQVCHFVTMCIFTCTGQH
ASVHLGQLDWYSWVPNAPCTMRLPPPTTKDATLETVMATLPNFHQASLQMSITWQLGRRQ
PVMVAVGQHEEEYFSGPEPKAVLKKFREELAALDKEIEIRNAKLDMPYEYLRPSVVENSV
AI
|
|
|
|---|
| BDBM50206257 |
|---|
| n/a |
|---|
| Name | BDBM50206257 |
|---|
| Synonyms: | CHEMBL387584 | CHEMBL450482 | halisulfate 1 | sodium (2R,3R)-9-(2,5-dihydroxyphenyl)-3,7-dimethyl-1-((1S,4aS,8aS)-2,5,5,8a-tetramethyl-1,4,4a,5,6,7,8,8a-octahydronaphthalen-1-yl)non-7-en-2-yl sulfate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H47O6S |
|---|
| Mol. Mass. | 547.767 |
|---|
| SMILES | C[C@H](CCC\C(C)=C\Cc1cc(O)ccc1O)[C@@H](C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS([O-])(=O)=O |r,c:22| |
|---|
| Structure | 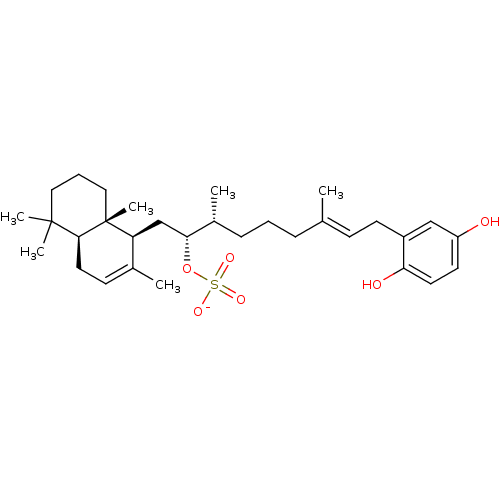
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Amagata, T; Whitman, S; Johnson, TA; Stessman, CC; Loo, CP; Lobkovsky, E; Clardy, J; Crews, P; Holman, TR Exploring sponge-derived terpenoids for their potency and selectivity against 12-human, 15-human, and 15-soybean lipoxygenases. J Nat Prod66:230-5 (2003) [PubMed] Article
Amagata, T; Whitman, S; Johnson, TA; Stessman, CC; Loo, CP; Lobkovsky, E; Clardy, J; Crews, P; Holman, TR Exploring sponge-derived terpenoids for their potency and selectivity against 12-human, 15-human, and 15-soybean lipoxygenases. J Nat Prod66:230-5 (2003) [PubMed] Article