

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Glutamate racemase | ||
| Ligand | BDBM50421083 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_842443 (CHEMBL2092116) | ||
| IC50 | 250±n/a nM | ||
| Citation |  Basarab, GS; Hill, P; Eyermann, CJ; Gowravaram, M; Käck, H; Osimoni, E Design of inhibitors of Helicobacter pylori glutamate racemase as selective antibacterial agents: incorporation of imidazoles onto a core pyrazolopyrimidinedione scaffold to improve bioavailabilty. Bioorg Med Chem Lett22:5600-7 (2012) [PubMed] Article Basarab, GS; Hill, P; Eyermann, CJ; Gowravaram, M; Käck, H; Osimoni, E Design of inhibitors of Helicobacter pylori glutamate racemase as selective antibacterial agents: incorporation of imidazoles onto a core pyrazolopyrimidinedione scaffold to improve bioavailabilty. Bioorg Med Chem Lett22:5600-7 (2012) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Glutamate racemase | |||
| Name: | Glutamate racemase | ||
| Synonyms: | MURI_HELPJ | glr | murI | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 28499.39 | ||
| Organism: | Helicobacter pylori J99 | ||
| Description: | ChEMBL_842443 | ||
| Residue: | 255 | ||
| Sequence: |
| ||
| BDBM50421083 | |||
| n/a | |||
| Name | BDBM50421083 | ||
| Synonyms: | CHEMBL2088118 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C27H23ClN8O2 | ||
| Mol. Mass. | 526.977 | ||
| SMILES | Cn1cc(nc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC=C)c(=O)c12)C#N |(12.66,-40.8,;14.16,-41.15,;15.32,-40.14,;16.64,-40.93,;16.3,-42.43,;14.76,-42.57,;13.96,-43.88,;14.87,-45.13,;16.41,-45.13,;17.18,-46.47,;16.41,-47.79,;17.18,-49.13,;18.72,-49.13,;19.49,-47.78,;21.01,-47.77,;21.77,-46.45,;20.99,-45.12,;21.75,-43.78,;19.47,-45.14,;18.72,-46.46,;13.96,-46.38,;12.5,-45.9,;11.17,-46.66,;11.17,-48.2,;9.83,-48.97,;8.3,-48.98,;9.07,-50.31,;9.84,-45.9,;8.51,-46.67,;9.84,-44.36,;8.5,-43.59,;8.5,-42.05,;7.16,-41.29,;11.17,-43.58,;11.17,-42.04,;12.5,-44.36,;18.05,-40.33,;19.47,-39.73,)| | ||
| Structure | 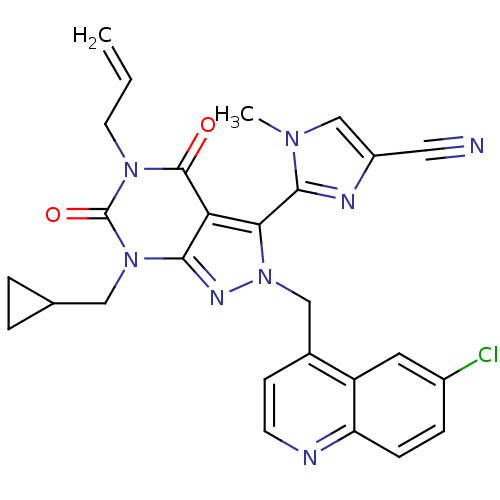
| ||