 Found 105 hits with Last Name = 'käck' and Initial = 'h'
Found 105 hits with Last Name = 'käck' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339997
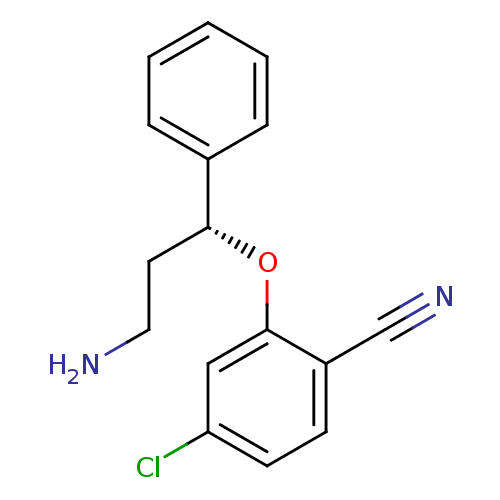
((R)-2-(3-amino-1-phenylpropoxy)-4-chlorobenzonitri...)Show InChI InChI=1S/C16H15ClN2O/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339998
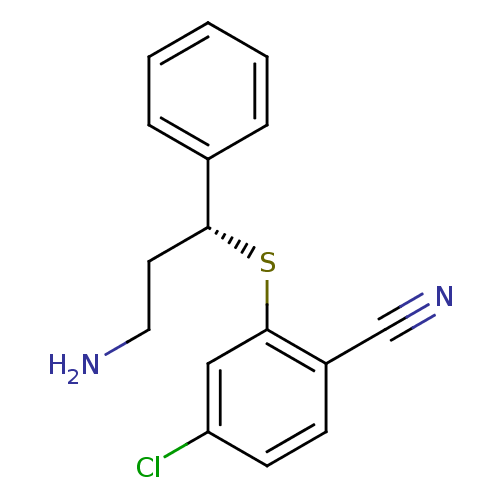
((R)-2-(3-amino-1-phenylpropylthio)-4-chlorobenzoni...)Show InChI InChI=1S/C16H15ClN2S/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340003

((R)-2-(3-amino-1-phenylpropylthio)-6-methylnicotin...)Show InChI InChI=1S/C16H17N3S/c1-12-7-8-14(11-18)16(19-12)20-15(9-10-17)13-5-3-2-4-6-13/h2-8,15H,9-10,17H2,1H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340004
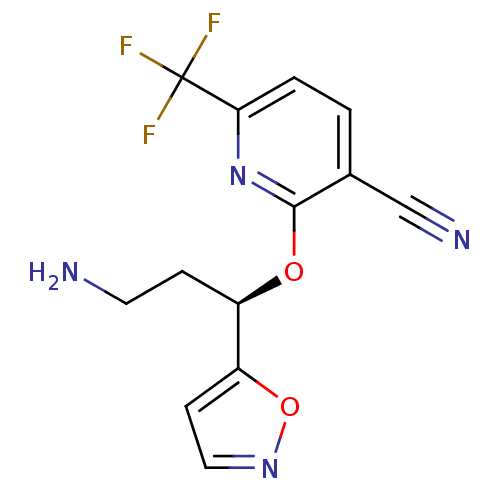
((R)-2-(3-amino-1-(isoxazol-5-yl)propoxy)-6-(triflu...)Show SMILES NCC[C@@H](Oc1nc(ccc1C#N)C(F)(F)F)c1ccno1 |r| Show InChI InChI=1S/C13H11F3N4O2/c14-13(15,16)11-2-1-8(7-18)12(20-11)21-9(3-5-17)10-4-6-19-22-10/h1-2,4,6,9H,3,5,17H2/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340002

(2-[[(1R)-3-Amino-1-phenylpropyl]oxy]-4-chloro-5-fl...)Show InChI InChI=1S/C16H14ClFN2O/c17-13-9-16(12(10-20)8-14(13)18)21-15(6-7-19)11-4-2-1-3-5-11/h1-5,8-9,15H,6-7,19H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339996

((R)-4-chloro-2-(3-(methylamino)-1-phenylpropoxy)be...)Show InChI InChI=1S/C17H17ClN2O/c1-20-10-9-16(13-5-3-2-4-6-13)21-17-11-15(18)8-7-14(17)12-19/h2-8,11,16,20H,9-10H2,1H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421064
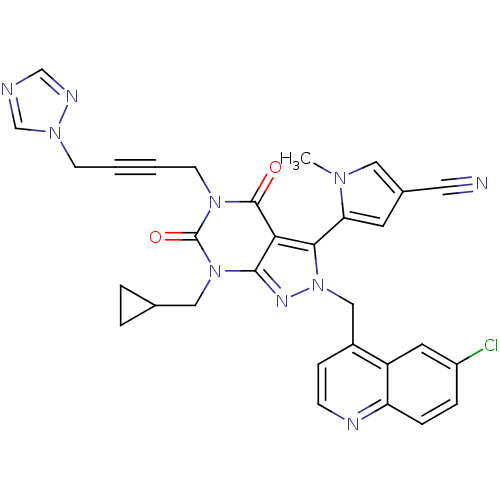
(CHEMBL2087855)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#CCn3cncn3)c(=O)c12)C#N |(34.36,2.12,;35.86,1.77,;37.02,2.78,;38.34,1.99,;38,.49,;36.46,.35,;35.67,-.96,;36.57,-2.21,;38.11,-2.21,;38.88,-3.54,;38.11,-4.87,;38.88,-6.21,;40.42,-6.21,;41.19,-4.86,;42.71,-4.85,;43.47,-3.53,;42.7,-2.2,;43.45,-.86,;41.17,-2.21,;40.42,-3.54,;35.67,-3.46,;34.2,-2.98,;32.87,-3.74,;32.87,-5.28,;31.54,-6.05,;30,-6.06,;30.77,-7.39,;31.54,-2.98,;30.21,-3.75,;31.54,-1.44,;30.21,-.67,;30.2,.87,;30.19,2.41,;30.18,3.95,;31.5,4.73,;32.91,4.12,;33.93,5.27,;33.15,6.6,;31.64,6.27,;32.87,-.66,;32.87,.88,;34.2,-1.44,;39.76,2.59,;41.17,3.19,)| Show InChI InChI=1S/C31H25ClN10O2/c1-38-15-21(14-33)12-26(38)28-27-29(37-42(28)17-22-8-9-35-25-7-6-23(32)13-24(22)25)41(16-20-4-5-20)31(44)40(30(27)43)11-3-2-10-39-19-34-18-36-39/h6-9,12-13,15,18-20H,4-5,10-11,16-17H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339995
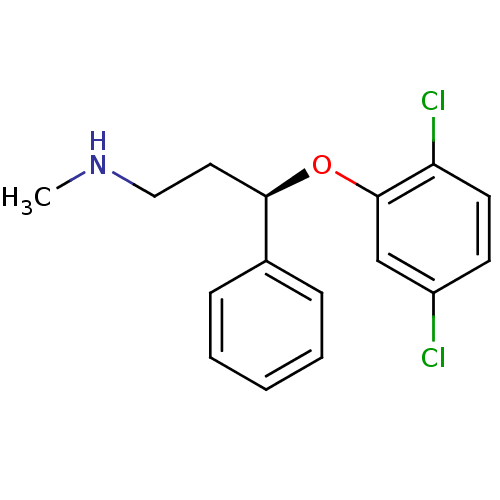
((R)-3-(2,5-dichlorophenoxy)-N-methyl-3-phenylpropa...)Show InChI InChI=1S/C16H17Cl2NO/c1-19-10-9-15(12-5-3-2-4-6-12)20-16-11-13(17)7-8-14(16)18/h2-8,11,15,19H,9-10H2,1H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421055

(CHEMBL1652588)Show SMILES Cn1cncc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#C)c(=O)c12 |(11.26,-9.68,;12.8,-9.68,;13.7,-8.44,;15.16,-8.91,;15.16,-10.45,;13.7,-10.92,;13.23,-12.37,;14.14,-13.62,;15.68,-13.62,;16.45,-14.96,;15.68,-16.29,;16.45,-17.63,;17.99,-17.63,;18.76,-16.28,;20.29,-16.27,;21.05,-14.94,;20.27,-13.61,;21.03,-12.27,;18.74,-13.63,;17.99,-14.95,;13.23,-14.87,;11.76,-14.4,;10.43,-15.16,;10.43,-16.7,;9.09,-17.47,;7.55,-17.48,;8.32,-18.81,;9.1,-14.39,;7.76,-15.17,;9.1,-12.85,;7.76,-12.08,;7.76,-10.54,;7.75,-9.01,;10.43,-12.07,;10.43,-10.53,;11.76,-12.85,)| Show InChI InChI=1S/C26H22ClN7O2/c1-3-10-32-25(35)22-23(21-12-28-15-31(21)2)34(30-24(22)33(26(32)36)13-16-4-5-16)14-17-8-9-29-20-7-6-18(27)11-19(17)20/h1,6-9,11-12,15-16H,4-5,10,13-14H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50215445
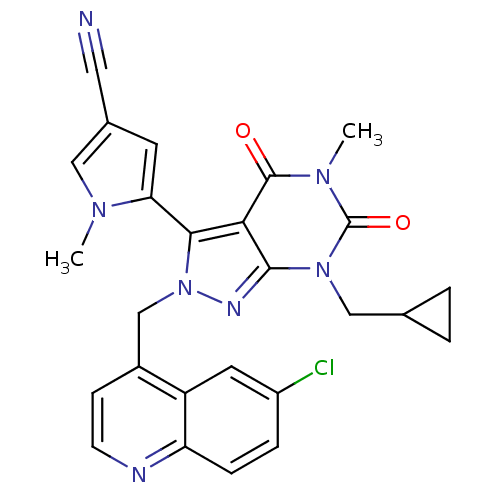
(5-(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropy...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(17.56,4.29,;19.1,4.29,;20,5.54,;21.47,5.06,;21.47,3.52,;20,3.04,;19.53,1.58,;20.45,.33,;21.99,.33,;22.76,-1.01,;21.98,-2.34,;22.75,-3.67,;24.29,-3.67,;25.06,-2.32,;26.59,-2.32,;27.35,-.99,;26.57,.34,;27.33,1.68,;25.05,.33,;24.29,-1,;19.53,-.93,;18.06,-.45,;16.72,-1.22,;16.71,-2.76,;15.38,-3.52,;13.84,-3.53,;14.61,-4.86,;15.38,-.45,;14.05,-1.22,;15.38,1.1,;14.05,1.87,;16.72,1.88,;16.71,3.42,;18.06,1.1,;22.71,5.96,;23.96,6.86,)| Show InChI InChI=1S/C26H22ClN7O2/c1-31-12-16(11-28)9-21(31)23-22-24(33(13-15-3-4-15)26(36)32(2)25(22)35)30-34(23)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,12,15H,3-4,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421065
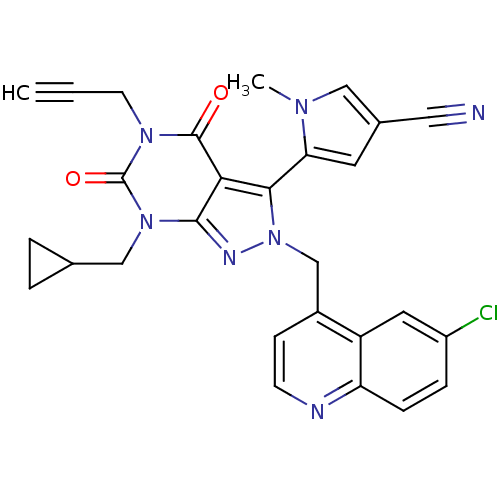
(CHEMBL2087856)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#C)c(=O)c12)C#N |(-4.86,-12.02,;-3.36,-12.36,;-2.2,-11.35,;-.87,-12.15,;-1.22,-13.65,;-2.76,-13.78,;-3.55,-15.1,;-2.65,-16.34,;-1.11,-16.34,;-.34,-17.68,;-1.11,-19.01,;-.34,-20.34,;1.2,-20.34,;1.97,-18.99,;3.49,-18.99,;4.25,-17.66,;3.48,-16.33,;4.24,-14.99,;1.95,-16.35,;1.2,-17.67,;-3.55,-17.59,;-5.02,-17.11,;-6.35,-17.87,;-6.35,-19.41,;-7.68,-20.18,;-9.22,-20.19,;-8.45,-21.53,;-7.68,-17.11,;-9.01,-17.88,;-7.68,-15.57,;-9.01,-14.81,;-9.02,-13.27,;-9.03,-11.72,;-6.35,-14.79,;-6.35,-13.25,;-5.02,-15.57,;.54,-11.55,;1.95,-10.94,)| Show InChI InChI=1S/C28H22ClN7O2/c1-3-10-34-27(37)24-25(23-11-18(13-30)14-33(23)2)36(32-26(24)35(28(34)38)15-17-4-5-17)16-19-8-9-31-22-7-6-20(29)12-21(19)22/h1,6-9,11-12,14,17H,4-5,10,15-16H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50262114
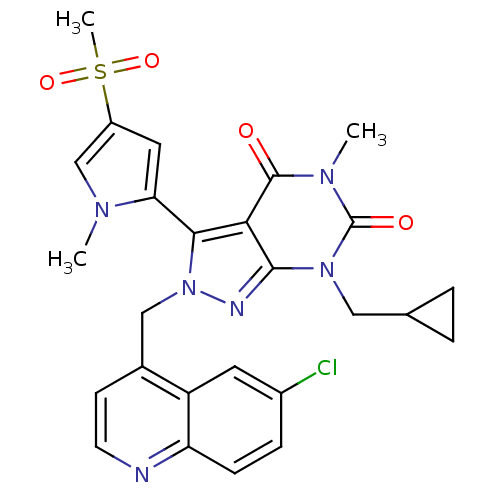
(2-((6-chloroquinolin-4-yl)methyl)-7-(cyclopropylme...)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)S(C)(=O)=O |(-3.7,2.05,;-2.16,2.05,;-1.26,3.29,;.2,2.82,;.21,1.29,;-1.26,.8,;-1.73,-.66,;-.82,-1.91,;.72,-1.91,;1.5,-3.25,;.72,-4.58,;1.49,-5.91,;3.04,-5.92,;3.8,-4.57,;5.33,-4.56,;6.1,-3.23,;5.31,-1.9,;6.07,-.56,;3.79,-1.92,;3.03,-3.24,;-1.73,-3.17,;-3.2,-2.69,;-4.55,-3.46,;-4.55,-5,;-5.89,-5.77,;-7.42,-5.77,;-6.66,-7.11,;-5.88,-2.69,;-7.22,-3.46,;-5.88,-1.14,;-7.22,-.37,;-4.55,-.36,;-4.55,1.18,;-3.2,-1.14,;1.45,3.73,;2.69,4.64,;.6,5.02,;2.3,2.45,)| Show InChI InChI=1S/C26H25ClN6O4S/c1-30-14-18(38(3,36)37)11-21(30)23-22-24(32(12-15-4-5-15)26(35)31(2)25(22)34)29-33(23)13-16-8-9-28-20-7-6-17(27)10-19(16)20/h6-11,14-15H,4-5,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421042
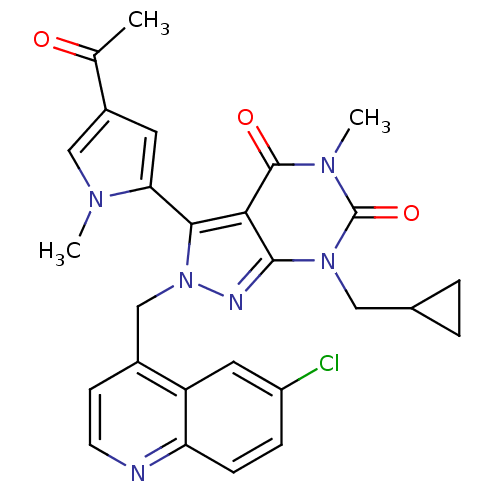
(CHEMBL2087619)Show SMILES CC(=O)c1cc(-c2n(Cc3ccnc4ccc(Cl)cc34)nc3n(CC4CC4)c(=O)n(C)c(=O)c23)n(C)c1 |(37.58,2.53,;36.35,3.45,;36.54,4.98,;34.93,2.85,;34.59,1.35,;33.05,1.22,;32.26,-.1,;33.16,-1.35,;34.7,-1.35,;35.47,-2.68,;34.7,-4.01,;35.47,-5.34,;37.01,-5.34,;37.78,-4,;39.3,-3.99,;40.06,-2.66,;39.29,-1.34,;40.04,0,;37.76,-1.35,;37.01,-2.67,;32.26,-2.59,;30.79,-2.12,;29.46,-2.88,;29.46,-4.42,;28.13,-5.19,;26.59,-5.19,;27.36,-6.53,;28.13,-2.12,;26.8,-2.89,;28.13,-.58,;26.8,.19,;29.46,.2,;29.46,1.74,;30.79,-.58,;32.45,2.63,;30.95,2.98,;33.61,3.64,)| Show InChI InChI=1S/C27H25ClN6O3/c1-15(35)18-10-22(31(2)13-18)24-23-25(33(12-16-4-5-16)27(37)32(3)26(23)36)30-34(24)14-17-8-9-29-21-7-6-19(28)11-20(17)21/h6-11,13,16H,4-5,12,14H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124535
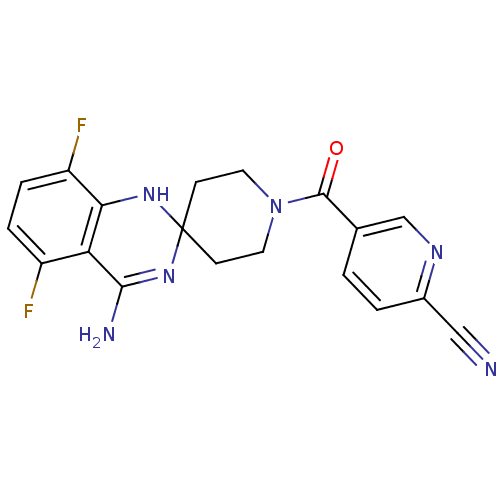
(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421086

(CHEMBL2087620)Show SMILES Cn1cncc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12 |(-4.9,-12.42,;-3.4,-12.77,;-2.23,-11.76,;-.91,-12.55,;-1.26,-14.05,;-2.79,-14.19,;-3.59,-15.5,;-2.68,-16.75,;-1.14,-16.75,;-.37,-18.08,;-1.15,-19.41,;-.38,-20.74,;1.16,-20.75,;1.93,-19.4,;3.46,-19.39,;4.22,-18.06,;3.44,-16.74,;4.2,-15.4,;1.91,-16.75,;1.16,-18.07,;-3.59,-18,;-5.06,-17.52,;-6.39,-18.28,;-6.39,-19.82,;-7.72,-20.59,;-9.26,-20.6,;-8.49,-21.93,;-7.72,-17.52,;-9.05,-18.29,;-7.72,-15.98,;-9.05,-15.21,;-6.39,-15.2,;-6.39,-13.66,;-5.06,-15.98,)| Show InChI InChI=1S/C24H22ClN7O2/c1-29-13-26-10-19(29)21-20-22(31(11-14-3-4-14)24(34)30(2)23(20)33)28-32(21)12-15-7-8-27-18-6-5-16(25)9-17(15)18/h5-10,13-14H,3-4,11-12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421066
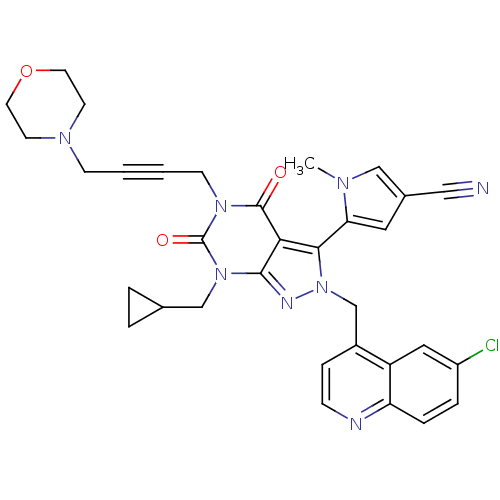
(CHEMBL2087857)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#CCN3CCOCC3)c(=O)c12)C#N |(14.03,-12.75,;15.53,-13.09,;16.69,-12.08,;18.01,-12.88,;17.66,-14.38,;16.13,-14.51,;15.33,-15.83,;16.24,-17.07,;17.78,-17.07,;18.55,-18.41,;17.78,-19.74,;18.55,-21.07,;20.09,-21.07,;20.85,-19.72,;22.38,-19.72,;23.14,-18.39,;22.36,-17.07,;23.12,-15.73,;20.84,-17.08,;20.09,-18.4,;15.33,-18.32,;13.87,-17.85,;12.54,-18.61,;12.54,-20.15,;11.2,-20.92,;9.66,-20.92,;10.43,-22.26,;11.21,-17.84,;9.88,-18.62,;11.21,-16.3,;9.87,-15.54,;9.87,-14,;9.86,-12.45,;9.84,-10.91,;11.17,-10.13,;12.5,-10.9,;13.82,-10.13,;13.82,-8.58,;12.48,-7.82,;11.14,-8.6,;12.54,-15.53,;12.54,-13.99,;13.87,-16.3,;19.42,-12.28,;20.84,-11.68,)| Show InChI InChI=1S/C33H31ClN8O3/c1-38-19-23(18-35)16-28(38)30-29-31(37-42(30)21-24-8-9-36-27-7-6-25(34)17-26(24)27)41(20-22-4-5-22)33(44)40(32(29)43)11-3-2-10-39-12-14-45-15-13-39/h6-9,16-17,19,22H,4-5,10-15,20-21H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340001
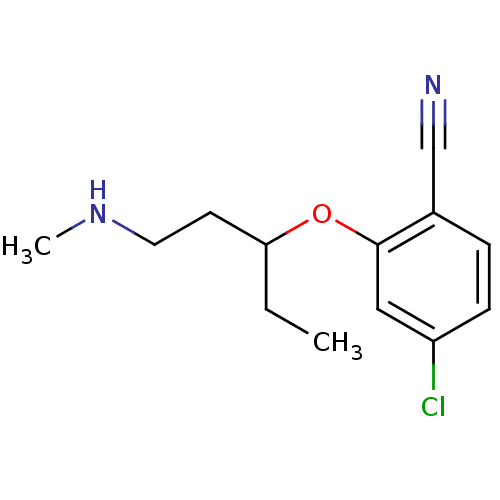
(CHEMBL1762478 | rac-4-chloro-2-(1-(methylamino)pen...)Show InChI InChI=1S/C13H17ClN2O/c1-3-12(6-7-16-2)17-13-8-11(14)5-4-10(13)9-15/h4-5,8,12,16H,3,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421056

(CHEMBL2087846)Show SMILES Cn1cncc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#CCO)c(=O)c12 |(30.7,-12.2,;32.2,-12.55,;33.36,-11.54,;34.68,-12.33,;34.33,-13.83,;32.8,-13.97,;32,-15.28,;32.91,-16.53,;34.45,-16.53,;35.22,-17.86,;34.45,-19.19,;35.22,-20.53,;36.76,-20.53,;37.52,-19.18,;39.05,-19.17,;39.81,-17.85,;39.03,-16.52,;39.79,-15.18,;37.51,-16.53,;36.75,-17.86,;32,-17.78,;30.54,-17.3,;29.21,-18.06,;29.21,-19.6,;27.87,-20.37,;26.33,-20.38,;27.1,-21.71,;27.88,-17.3,;26.54,-18.07,;27.88,-15.76,;26.54,-14.99,;26.54,-13.45,;26.52,-11.91,;26.51,-10.37,;27.84,-9.59,;29.21,-14.98,;29.21,-13.44,;30.54,-15.76,)| Show InChI InChI=1S/C27H24ClN7O3/c1-32-16-29-13-22(32)24-23-25(31-35(24)15-18-8-9-30-21-7-6-19(28)12-20(18)21)34(14-17-4-5-17)27(38)33(26(23)37)10-2-3-11-36/h6-9,12-13,16-17,36H,4-5,10-11,14-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421067
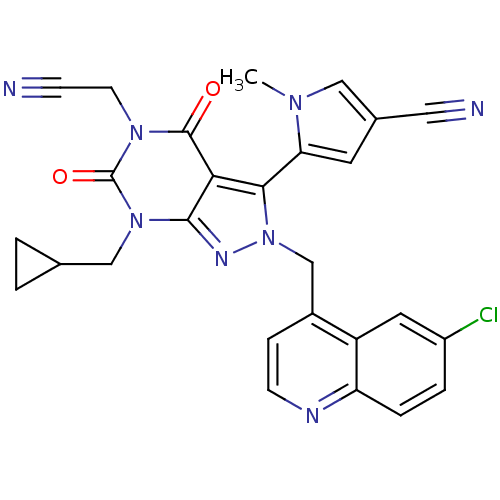
(CHEMBL2087858)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#N)c(=O)c12)C#N |(33.54,-11.99,;35.04,-12.34,;36.2,-11.33,;37.52,-12.12,;37.17,-13.62,;35.64,-13.76,;34.84,-15.07,;35.75,-16.32,;37.29,-16.32,;38.06,-17.65,;37.29,-18.98,;38.06,-20.32,;39.6,-20.32,;40.36,-18.97,;41.89,-18.96,;42.65,-17.64,;41.87,-16.31,;42.63,-14.97,;40.35,-16.32,;39.59,-17.65,;34.84,-17.57,;33.38,-17.09,;32.05,-17.85,;32.05,-19.39,;30.71,-20.16,;29.17,-20.17,;29.94,-21.5,;30.72,-17.09,;29.39,-17.86,;30.72,-15.55,;29.38,-14.78,;29.38,-13.24,;29.37,-11.71,;32.05,-14.77,;32.05,-13.23,;33.38,-15.55,;38.93,-11.52,;40.35,-10.92,)| Show InChI InChI=1S/C27H21ClN8O2/c1-33-13-17(12-30)10-22(33)24-23-25(35(14-16-2-3-16)27(38)34(9-7-29)26(23)37)32-36(24)15-18-6-8-31-21-5-4-19(28)11-20(18)21/h4-6,8,10-11,13,16H,2-3,9,14-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421057
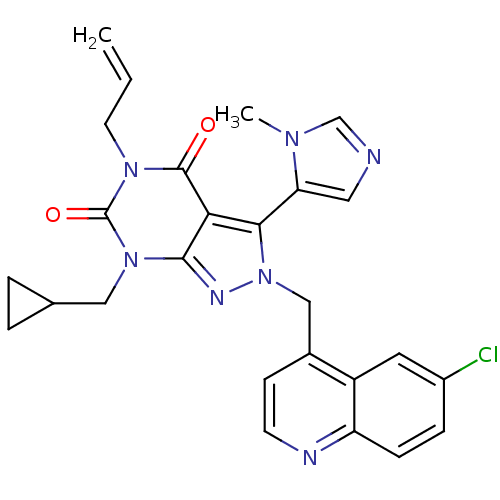
(CHEMBL2087847)Show SMILES Cn1cncc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC=C)c(=O)c12 |(-3.64,-25.96,;-2.14,-26.3,;-.97,-25.29,;.35,-26.08,;-0,-27.59,;-1.53,-27.72,;-2.33,-29.04,;-1.42,-30.28,;.12,-30.28,;.89,-31.62,;.11,-32.95,;.88,-34.28,;2.42,-34.28,;3.19,-32.93,;4.72,-32.93,;5.48,-31.6,;4.7,-30.27,;5.46,-28.93,;3.17,-30.29,;2.42,-31.61,;-2.33,-31.53,;-3.8,-31.05,;-5.13,-31.81,;-5.13,-33.35,;-6.46,-34.12,;-8,-34.13,;-7.23,-35.46,;-6.46,-31.05,;-7.79,-31.82,;-6.46,-29.51,;-7.79,-28.75,;-7.8,-27.21,;-9.13,-26.44,;-5.13,-28.73,;-5.13,-27.19,;-3.8,-29.51,)| Show InChI InChI=1S/C26H24ClN7O2/c1-3-10-32-25(35)22-23(21-12-28-15-31(21)2)34(30-24(22)33(26(32)36)13-16-4-5-16)14-17-8-9-29-20-7-6-18(27)11-19(17)20/h3,6-9,11-12,15-16H,1,4-5,10,13-14H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421068

(CHEMBL2087859)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#CCO)c(=O)c12)C#N |(-5.1,-25.48,;-3.6,-25.83,;-2.44,-24.82,;-1.12,-25.61,;-1.46,-27.11,;-3,-27.24,;-3.79,-28.56,;-2.89,-29.81,;-1.35,-29.81,;-.58,-31.14,;-1.35,-32.47,;-.58,-33.8,;.96,-33.8,;1.73,-32.46,;3.25,-32.45,;4.01,-31.12,;3.24,-29.8,;3.99,-28.46,;1.71,-29.81,;.96,-31.13,;-3.79,-31.05,;-5.26,-30.58,;-6.59,-31.34,;-6.59,-32.88,;-7.92,-33.65,;-9.46,-33.66,;-8.69,-34.99,;-7.92,-30.58,;-9.25,-31.35,;-7.92,-29.04,;-9.25,-28.27,;-9.26,-26.73,;-9.27,-25.19,;-9.28,-23.65,;-7.96,-22.86,;-6.59,-28.26,;-6.59,-26.72,;-5.26,-29.04,;.3,-25.01,;1.71,-24.41,)| Show InChI InChI=1S/C29H24ClN7O3/c1-34-15-19(14-31)12-24(34)26-25-27(33-37(26)17-20-8-9-32-23-7-6-21(30)13-22(20)23)36(16-18-4-5-18)29(40)35(28(25)39)10-2-3-11-38/h6-9,12-13,15,18,38H,4-5,10-11,16-17H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421044
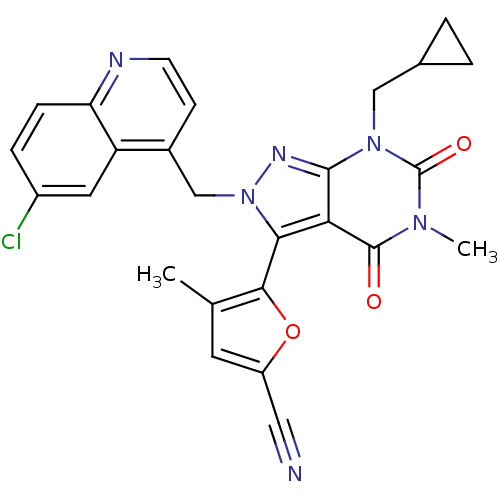
(CHEMBL2087622)Show SMILES Cc1cc(oc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(31.68,-12.9,;33.19,-13.24,;34.35,-12.23,;35.67,-13.02,;35.32,-14.53,;33.79,-14.66,;32.99,-15.98,;33.9,-17.22,;35.44,-17.22,;36.21,-18.56,;35.43,-19.89,;36.2,-21.22,;37.75,-21.22,;38.51,-19.87,;40.04,-19.87,;40.8,-18.54,;40.02,-17.21,;40.78,-15.87,;38.49,-17.23,;37.74,-18.55,;32.99,-18.47,;31.52,-17.99,;30.19,-18.75,;30.19,-20.29,;28.86,-21.06,;27.32,-21.07,;28.09,-22.4,;28.87,-17.99,;27.53,-18.76,;28.87,-16.45,;27.53,-15.69,;30.19,-15.67,;30.19,-14.13,;31.52,-16.45,;37.08,-12.42,;38.5,-11.82,)| Show InChI InChI=1S/C26H21ClN6O3/c1-14-9-18(11-28)36-23(14)22-21-24(32(12-15-3-4-15)26(35)31(2)25(21)34)30-33(22)13-16-7-8-29-20-6-5-17(27)10-19(16)20/h5-10,15H,3-4,12-13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421069

(CHEMBL2087860)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12)C#N |(14.84,-25.21,;16.35,-25.56,;17.51,-24.54,;18.83,-25.34,;18.48,-26.84,;16.95,-26.97,;16.15,-28.29,;17.06,-29.54,;18.6,-29.54,;19.37,-30.87,;18.59,-32.2,;19.36,-33.53,;20.91,-33.53,;21.67,-32.19,;23.2,-32.18,;23.96,-30.85,;23.18,-29.53,;23.94,-28.19,;21.66,-29.54,;20.9,-30.86,;16.15,-30.78,;14.68,-30.31,;13.35,-31.07,;13.35,-32.61,;12.02,-33.38,;10.48,-33.38,;11.25,-34.72,;12.03,-30.3,;10.69,-31.08,;12.03,-28.76,;10.69,-28,;10.68,-26.46,;11.44,-25.13,;9.9,-25.13,;13.35,-27.99,;13.35,-26.45,;14.68,-28.76,;20.24,-24.74,;21.66,-24.14,)| Show InChI InChI=1S/C29H26ClN7O2/c1-34-13-19(12-31)10-24(34)26-25-27(33-37(26)16-20-8-9-32-23-7-6-21(30)11-22(20)23)35(14-17-2-3-17)29(39)36(28(25)38)15-18-4-5-18/h6-11,13,17-18H,2-5,14-16H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421058
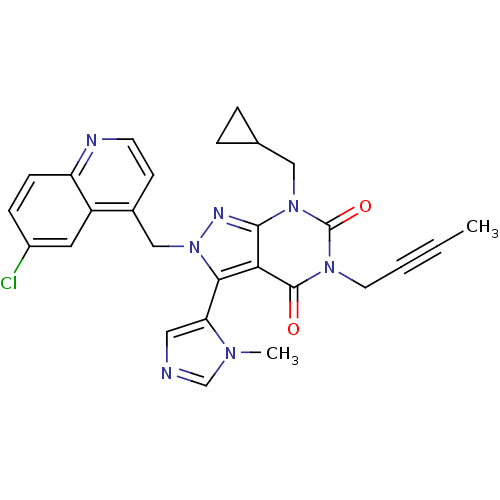
(CHEMBL2087848)Show SMILES CC#CCn1c(=O)n(CC2CC2)c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3cncn3C)c2c1=O |(9.77,-25.41,;9.78,-26.95,;9.8,-28.49,;9.8,-30.03,;11.14,-30.8,;11.14,-32.34,;9.81,-33.11,;12.47,-33.1,;12.47,-34.64,;11.13,-35.41,;9.59,-35.42,;10.36,-36.75,;13.8,-32.34,;15.26,-32.82,;16.17,-31.57,;17.71,-31.57,;18.48,-32.9,;17.71,-34.23,;18.48,-35.57,;20.02,-35.57,;20.78,-34.22,;22.31,-34.21,;23.07,-32.89,;22.29,-31.56,;23.05,-30.22,;20.77,-31.57,;20.01,-32.9,;15.26,-30.32,;16.06,-29.01,;17.59,-28.87,;17.94,-27.37,;16.62,-26.58,;15.46,-27.59,;13.96,-27.24,;13.8,-30.8,;12.47,-30.02,;12.47,-28.48,)| Show InChI InChI=1S/C27H24ClN7O2/c1-3-4-11-33-26(36)23-24(22-13-29-16-32(22)2)35(31-25(23)34(27(33)37)14-17-5-6-17)15-18-9-10-30-21-8-7-19(28)12-20(18)21/h7-10,12-13,16-17H,5-6,11,14-15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421059
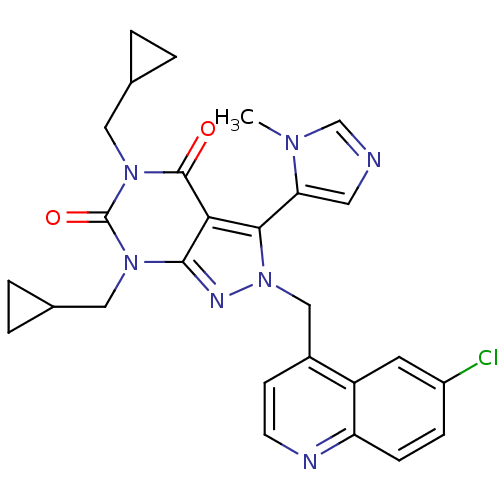
(CHEMBL2087849)Show SMILES Cn1cncc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 |(33.55,-26.78,;35.05,-27.13,;36.21,-26.12,;37.53,-26.91,;37.19,-28.41,;35.65,-28.54,;34.86,-29.86,;35.76,-31.11,;37.3,-31.11,;38.07,-32.44,;37.3,-33.77,;38.07,-35.1,;39.61,-35.1,;40.38,-33.76,;41.9,-33.75,;42.66,-32.42,;41.89,-31.1,;42.65,-29.76,;40.36,-31.11,;39.61,-32.43,;34.86,-32.35,;33.39,-31.88,;32.06,-32.64,;32.06,-34.18,;30.73,-34.95,;29.19,-34.95,;29.96,-36.29,;30.73,-31.88,;29.4,-32.65,;30.73,-30.34,;29.4,-29.57,;29.39,-28.03,;30.15,-26.7,;28.61,-26.7,;32.06,-29.56,;32.06,-28.02,;33.39,-30.34,)| Show InChI InChI=1S/C27H26ClN7O2/c1-32-15-29-11-22(32)24-23-25(31-35(24)14-18-8-9-30-21-7-6-19(28)10-20(18)21)33(12-16-2-3-16)27(37)34(26(23)36)13-17-4-5-17/h6-11,15-17H,2-5,12-14H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421070

(CHEMBL2087861)Show SMILES CCn1c(=O)n(CC2CC2)c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3cc(cn3C)C#N)c2c1=O |(30.23,-27.35,;30.24,-28.89,;31.57,-29.66,;31.57,-31.2,;30.24,-31.97,;32.9,-31.96,;32.9,-33.5,;31.57,-34.27,;30.03,-34.28,;30.8,-35.61,;34.23,-31.2,;35.7,-31.68,;36.6,-30.43,;38.14,-30.43,;38.91,-31.76,;38.14,-33.09,;38.91,-34.43,;40.45,-34.43,;41.22,-33.08,;42.74,-33.07,;43.5,-31.75,;42.73,-30.42,;43.49,-29.08,;41.2,-30.43,;40.45,-31.76,;35.7,-29.18,;36.49,-27.87,;38.03,-27.73,;38.37,-26.23,;37.05,-25.44,;35.89,-26.45,;34.39,-26.1,;39.79,-25.63,;41.2,-25.03,;34.23,-29.66,;32.9,-28.88,;32.9,-27.34,)| Show InChI InChI=1S/C27H24ClN7O2/c1-3-33-26(36)23-24(22-10-17(12-29)13-32(22)2)35(31-25(23)34(27(33)37)14-16-4-5-16)15-18-8-9-30-21-7-6-19(28)11-20(18)21/h6-11,13,16H,3-5,14-15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421071
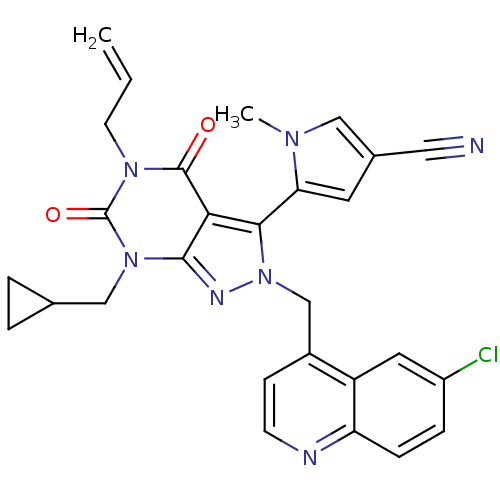
(CHEMBL2087862)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC=C)c(=O)c12)C#N |(-4.42,-41.12,;-2.92,-41.47,;-1.76,-40.46,;-.44,-41.25,;-.79,-42.75,;-2.32,-42.89,;-3.12,-44.2,;-2.21,-45.45,;-.67,-45.45,;.1,-46.78,;-.67,-48.11,;.1,-49.45,;1.64,-49.45,;2.4,-48.1,;3.93,-48.09,;4.69,-46.77,;3.91,-45.44,;4.67,-44.1,;2.39,-45.45,;1.64,-46.78,;-3.12,-46.7,;-4.58,-46.22,;-5.91,-46.98,;-5.91,-48.52,;-7.25,-49.29,;-8.79,-49.3,;-8.02,-50.63,;-7.24,-46.22,;-8.57,-46.99,;-7.24,-44.68,;-8.58,-43.91,;-8.58,-42.37,;-9.92,-41.61,;-5.91,-43.9,;-5.91,-42.36,;-4.58,-44.68,;.97,-40.65,;2.39,-40.05,)| Show InChI InChI=1S/C28H24ClN7O2/c1-3-10-34-27(37)24-25(23-11-18(13-30)14-33(23)2)36(32-26(24)35(28(34)38)15-17-4-5-17)16-19-8-9-31-22-7-6-20(29)12-21(19)22/h3,6-9,11-12,14,17H,1,4-5,10,15-16H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339999
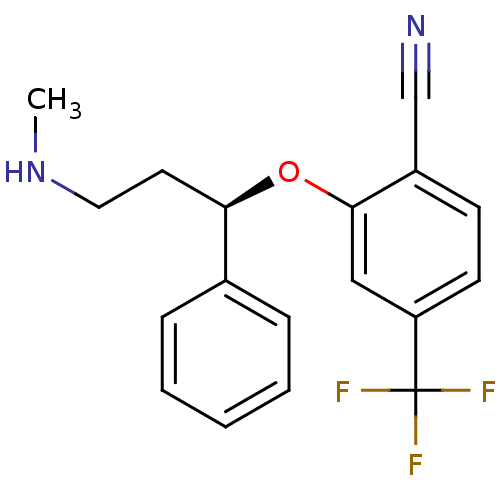
((R)-2-(3-(methylamino)-1-phenylpropoxy)-4-(trifluo...)Show SMILES CNCC[C@@H](Oc1cc(ccc1C#N)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C18H17F3N2O/c1-23-10-9-16(13-5-3-2-4-6-13)24-17-11-15(18(19,20)21)8-7-14(17)12-22/h2-8,11,16,23H,9-10H2,1H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421060
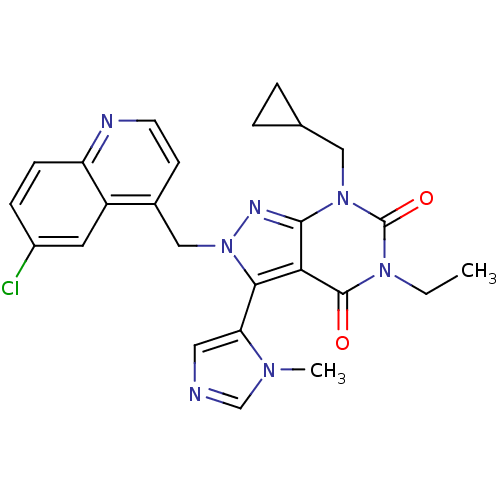
(CHEMBL2087850)Show SMILES CCn1c(=O)n(CC2CC2)c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3cncn3C)c2c1=O |(-8.44,-41.43,;-8.44,-42.97,;-7.1,-43.74,;-7.1,-45.28,;-8.43,-46.05,;-5.77,-46.04,;-5.77,-47.58,;-7.11,-48.35,;-8.65,-48.36,;-7.88,-49.69,;-4.44,-45.28,;-2.98,-45.76,;-2.07,-44.51,;-.53,-44.51,;.24,-45.84,;-.53,-47.17,;.24,-48.51,;1.78,-48.51,;2.54,-47.16,;4.07,-47.15,;4.83,-45.83,;4.05,-44.5,;4.81,-43.16,;2.53,-44.51,;1.78,-45.84,;-2.98,-43.26,;-2.18,-41.95,;-.65,-41.81,;-.3,-40.31,;-1.62,-39.52,;-2.78,-40.53,;-4.28,-40.18,;-4.44,-43.74,;-5.77,-42.96,;-5.77,-41.42,)| Show InChI InChI=1S/C25H24ClN7O2/c1-3-31-24(34)21-22(20-11-27-14-30(20)2)33(29-23(21)32(25(31)35)12-15-4-5-15)13-16-8-9-28-19-7-6-17(26)10-18(16)19/h6-11,14-15H,3-5,12-13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421072
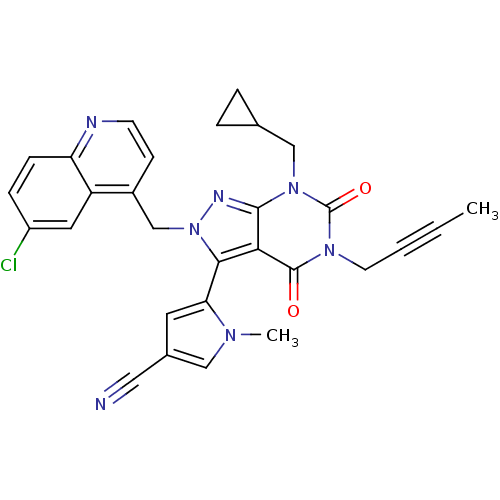
(CHEMBL2087863)Show SMILES CC#CCn1c(=O)n(CC2CC2)c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3cc(cn3C)C#N)c2c1=O |(10.14,-38.47,;10.15,-40.01,;10.16,-41.56,;10.17,-43.1,;11.5,-43.86,;11.5,-45.4,;10.17,-46.17,;12.83,-46.16,;12.83,-47.7,;11.5,-48.47,;9.96,-48.48,;10.73,-49.82,;14.16,-45.4,;15.63,-45.88,;16.54,-44.63,;18.08,-44.63,;18.85,-45.97,;18.07,-47.3,;18.84,-48.63,;20.38,-48.63,;21.15,-47.28,;22.68,-47.28,;23.44,-45.95,;22.66,-44.62,;23.42,-43.28,;21.13,-44.64,;20.38,-45.96,;15.63,-43.39,;16.43,-42.07,;17.96,-41.94,;18.31,-40.44,;16.99,-39.64,;15.82,-40.65,;14.32,-40.31,;19.72,-39.84,;21.14,-39.23,;14.16,-43.86,;12.83,-43.08,;12.83,-41.54,)| Show InChI InChI=1S/C29H24ClN7O2/c1-3-4-11-35-28(38)25-26(24-12-19(14-31)15-34(24)2)37(33-27(25)36(29(35)39)16-18-5-6-18)17-20-9-10-32-23-8-7-21(30)13-22(20)23/h7-10,12-13,15,18H,5-6,11,16-17H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421088

(CHEMBL2087851)Show SMILES Cn1cncc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C3CC3)c(=O)c12 |(14.08,-41.22,;15.58,-41.57,;16.75,-40.56,;18.07,-41.35,;17.72,-42.85,;16.18,-42.99,;15.39,-44.3,;16.29,-45.55,;17.83,-45.55,;18.6,-46.89,;17.83,-48.21,;18.6,-49.55,;20.14,-49.55,;20.91,-48.2,;22.44,-48.19,;23.2,-46.87,;22.42,-45.54,;23.18,-44.2,;20.89,-45.55,;20.14,-46.88,;15.39,-46.8,;13.92,-46.32,;12.59,-47.08,;12.59,-48.62,;11.26,-49.39,;9.72,-49.4,;10.49,-50.73,;11.26,-46.32,;9.93,-47.09,;11.26,-44.78,;9.93,-44.01,;9.15,-42.69,;8.38,-44.02,;12.59,-44,;12.59,-42.46,;13.92,-44.78,)| Show InChI InChI=1S/C26H24ClN7O2/c1-31-14-28-11-21(31)23-22-24(32(12-15-2-3-15)26(36)34(25(22)35)18-5-6-18)30-33(23)13-16-8-9-29-20-7-4-17(27)10-19(16)20/h4,7-11,14-15,18H,2-3,5-6,12-13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421073
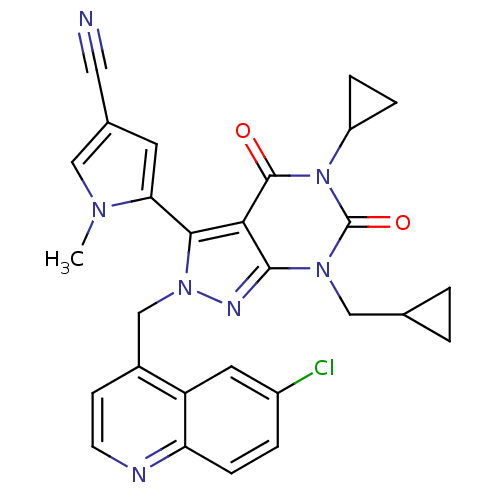
(CHEMBL2087864)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C3CC3)c(=O)c12)C#N |(33.45,-40.51,;34.95,-40.86,;36.11,-39.85,;37.43,-40.64,;37.09,-42.14,;35.55,-42.27,;34.76,-43.59,;35.66,-44.84,;37.2,-44.84,;37.97,-46.17,;37.2,-47.5,;37.97,-48.83,;39.51,-48.83,;40.28,-47.49,;41.8,-47.48,;42.56,-46.15,;41.79,-44.83,;42.55,-43.49,;40.26,-44.84,;39.51,-46.16,;34.76,-46.08,;33.29,-45.61,;31.96,-46.37,;31.96,-47.91,;30.63,-48.68,;29.09,-48.68,;29.86,-50.02,;30.63,-45.61,;29.3,-46.38,;30.63,-44.07,;29.3,-43.3,;28.52,-41.97,;27.75,-43.31,;31.96,-43.29,;31.96,-41.75,;33.29,-44.07,;38.85,-40.04,;40.26,-39.44,)| Show InChI InChI=1S/C28H24ClN7O2/c1-33-13-17(12-30)10-23(33)25-24-26(34(14-16-2-3-16)28(38)36(27(24)37)20-5-6-20)32-35(25)15-18-8-9-31-22-7-4-19(29)11-21(18)22/h4,7-11,13,16,20H,2-3,5-6,14-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421045

(CHEMBL2087623)Show SMILES CC(C)Cn1c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3cc(cn3C)C#N)c2c(=O)n(C)c1=O |(-8.95,-32.97,;-7.61,-33.74,;-7.62,-35.27,;-6.28,-32.97,;-6.28,-31.43,;-4.95,-30.67,;-3.48,-31.15,;-2.58,-29.9,;-1.04,-29.9,;-.27,-31.23,;-1.04,-32.56,;-.27,-33.89,;1.27,-33.9,;2.04,-32.55,;3.57,-32.54,;4.33,-31.21,;3.55,-29.89,;4.31,-28.55,;2.02,-29.9,;1.27,-31.22,;-3.48,-28.65,;-2.69,-27.33,;-1.15,-27.2,;-.8,-25.7,;-2.13,-24.91,;-3.29,-25.92,;-4.79,-25.57,;.61,-25.11,;2.03,-24.51,;-4.95,-29.13,;-6.28,-28.35,;-6.28,-26.81,;-7.61,-29.13,;-8.94,-28.36,;-7.61,-30.67,;-8.94,-31.44,)| Show InChI InChI=1S/C26H24ClN7O2/c1-15(2)12-33-24-22(25(35)32(4)26(33)36)23(21-9-16(11-28)13-31(21)3)34(30-24)14-17-7-8-29-20-6-5-18(27)10-19(17)20/h5-10,13,15H,12,14H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421043

(CHEMBL2087621)Show SMILES Cn1cc(nc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(12.52,-13.08,;14.02,-13.42,;15.18,-12.41,;16.51,-13.21,;16.16,-14.71,;14.62,-14.84,;13.83,-16.16,;14.73,-17.4,;16.27,-17.4,;17.04,-18.74,;16.27,-20.07,;17.04,-21.4,;18.58,-21.4,;19.35,-20.05,;20.88,-20.05,;21.64,-18.72,;20.86,-17.4,;21.62,-16.06,;19.33,-17.41,;18.58,-18.73,;13.83,-18.65,;12.36,-18.18,;11.03,-18.94,;11.03,-20.48,;9.7,-21.25,;8.16,-21.25,;8.93,-22.59,;9.7,-18.17,;8.37,-18.95,;9.7,-16.63,;8.37,-15.87,;11.03,-15.86,;11.03,-14.32,;12.36,-16.63,;17.92,-12.61,;19.33,-12,)| Show InChI InChI=1S/C25H21ClN8O2/c1-31-13-17(10-27)29-23(31)21-20-22(33(11-14-3-4-14)25(36)32(2)24(20)35)30-34(21)12-15-7-8-28-19-6-5-16(26)9-18(15)19/h5-9,13-14H,3-4,11-12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421074
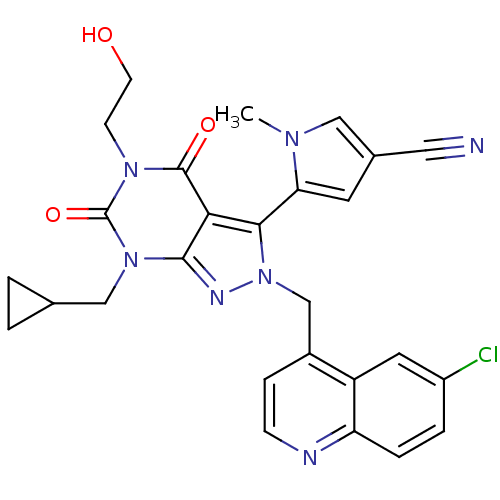
(CHEMBL2087865)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CCO)c(=O)c12)C#N |(-3.63,3.59,;-2.13,3.24,;-.97,4.25,;.35,3.46,;.01,1.96,;-1.53,1.82,;-2.32,.51,;-1.42,-.74,;.12,-.74,;.89,-2.07,;.12,-3.4,;.89,-4.74,;2.43,-4.74,;3.2,-3.39,;4.72,-3.38,;5.48,-2.06,;4.71,-.73,;5.47,.61,;3.18,-.74,;2.43,-2.07,;-2.32,-1.99,;-3.79,-1.51,;-5.12,-2.27,;-5.12,-3.81,;-6.45,-4.58,;-7.99,-4.59,;-7.22,-5.92,;-6.45,-1.51,;-7.78,-2.28,;-6.45,.03,;-7.78,.8,;-7.79,2.34,;-9.13,3.1,;-5.12,.81,;-5.12,2.35,;-3.79,.03,;1.77,4.06,;3.18,4.66,)| Show InChI InChI=1S/C27H24ClN7O3/c1-32-13-17(12-29)10-22(32)24-23-25(34(14-16-2-3-16)27(38)33(8-9-36)26(23)37)31-35(24)15-18-6-7-30-21-5-4-19(28)11-20(18)21/h4-7,10-11,13,16,36H,2-3,8-9,14-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421080

(CHEMBL2088115)Show SMILES Cn1cc(nc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#C)c(=O)c12)C#N |(12.9,-27.98,;14.4,-28.32,;15.56,-27.31,;16.88,-28.11,;16.54,-29.61,;15,-29.74,;14.2,-31.06,;15.11,-32.3,;16.65,-32.3,;17.42,-33.64,;16.65,-34.97,;17.42,-36.3,;18.96,-36.3,;19.73,-34.95,;21.25,-34.95,;22.01,-33.62,;21.23,-32.3,;21.99,-30.96,;19.71,-32.31,;18.96,-33.63,;14.21,-33.55,;12.74,-33.08,;11.41,-33.84,;11.41,-35.38,;10.07,-36.15,;8.54,-36.15,;9.31,-37.49,;10.08,-33.07,;8.75,-33.85,;10.08,-31.53,;8.74,-30.77,;8.74,-29.23,;8.73,-27.68,;11.41,-30.76,;11.41,-29.22,;12.74,-31.53,;18.29,-27.51,;19.71,-26.91,)| Show InChI InChI=1S/C27H21ClN8O2/c1-3-10-34-26(37)22-23(25-31-19(12-29)15-33(25)2)36(32-24(22)35(27(34)38)13-16-4-5-16)14-17-8-9-30-21-7-6-18(28)11-20(17)21/h1,6-9,11,15-16H,4-5,10,13-14H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50124535

(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340000
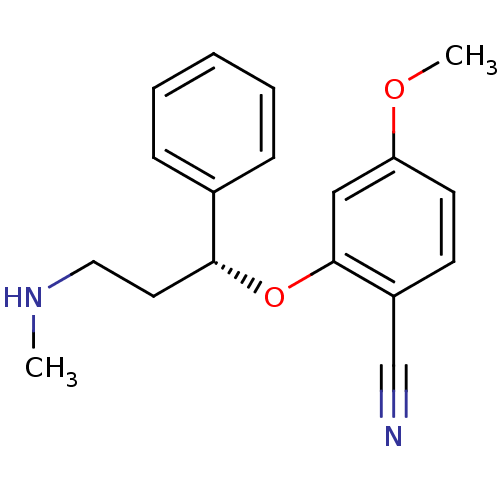
((R)-4-methoxy-2-(3-(methylamino)-1-phenylpropoxy)b...)Show InChI InChI=1S/C18H20N2O2/c1-20-11-10-17(14-6-4-3-5-7-14)22-18-12-16(21-2)9-8-15(18)13-19/h3-9,12,17,20H,10-11H2,1-2H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421085
(CHEMBL2088120)Show SMILES CC(C)Cn1c2nn(Cc3cccc4ccccc34)c(-c3cncn3C)c2c(=O)n(C)c1=O |(10.35,-3,;11.12,-1.67,;10.35,-.34,;12.66,-1.67,;13.43,-.33,;14.97,-.33,;16,-1.47,;17.41,-.84,;18.74,-1.6,;18.75,-3.14,;17.42,-3.91,;17.42,-5.46,;18.75,-6.23,;20.09,-5.45,;21.42,-6.22,;22.76,-5.44,;22.75,-3.89,;21.41,-3.13,;20.08,-3.91,;17.24,.69,;18.46,1.62,;19.94,1.17,;20.82,2.43,;19.9,3.66,;18.44,3.16,;17.18,4.05,;15.73,1.01,;14.97,2.33,;15.74,3.66,;13.43,2.34,;12.67,3.67,;12.66,1,;11.12,1.01,)| Show InChI InChI=1S/C25H26N6O2/c1-16(2)13-30-23-21(24(32)29(4)25(30)33)22(20-12-26-15-28(20)3)31(27-23)14-18-10-7-9-17-8-5-6-11-19(17)18/h5-12,15-16H,13-14H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421075
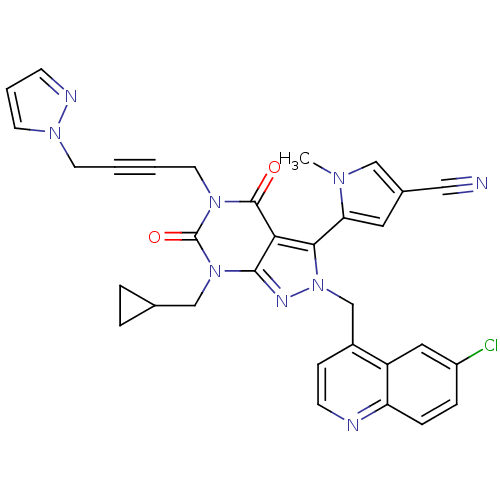
(CHEMBL2087866)Show SMILES Cn1cc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#CCn3cccn3)c(=O)c12)C#N |(13.57,2.5,;15.07,2.15,;16.23,3.16,;17.55,2.37,;17.21,.87,;15.67,.73,;14.87,-.58,;15.78,-1.83,;17.32,-1.83,;18.09,-3.16,;17.32,-4.49,;18.09,-5.82,;19.63,-5.83,;20.39,-4.48,;21.92,-4.47,;22.68,-3.14,;21.9,-1.82,;22.66,-.48,;20.38,-1.83,;19.63,-3.16,;14.87,-3.08,;13.41,-2.6,;12.08,-3.36,;12.08,-4.9,;10.74,-5.67,;9.21,-5.68,;9.98,-7.01,;10.75,-2.6,;9.42,-3.37,;10.75,-1.06,;9.41,-.29,;9.41,1.25,;9.4,2.79,;9.38,4.33,;10.71,5.11,;10.85,6.65,;12.35,6.98,;13.13,5.65,;12.11,4.5,;12.08,-.28,;12.08,1.26,;13.41,-1.06,;18.96,2.97,;20.38,3.57,)| Show InChI InChI=1S/C32H26ClN9O2/c1-38-18-22(17-34)15-27(38)29-28-30(37-42(29)20-23-9-11-35-26-8-7-24(33)16-25(23)26)41(19-21-5-6-21)32(44)40(31(28)43)14-3-2-12-39-13-4-10-36-39/h4,7-11,13,15-16,18,21H,5-6,12,14,19-20H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421081

(CHEMBL2088116)Show SMILES Cn1cc(nc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12)C#N |(31.96,-27.17,;33.46,-27.52,;34.62,-26.51,;35.94,-27.3,;35.6,-28.8,;34.06,-28.94,;33.26,-30.25,;34.17,-31.5,;35.71,-31.5,;36.48,-32.83,;35.71,-34.16,;36.48,-35.5,;38.02,-35.5,;38.79,-34.15,;40.31,-34.14,;41.07,-32.81,;40.3,-31.49,;41.05,-30.15,;38.77,-31.5,;38.02,-32.83,;33.27,-32.75,;31.8,-32.27,;30.47,-33.03,;30.47,-34.57,;29.13,-35.34,;27.6,-35.35,;28.37,-36.68,;29.14,-32.27,;27.81,-33.04,;29.14,-30.73,;27.8,-29.96,;27.8,-28.42,;28.56,-27.09,;27.02,-27.09,;30.47,-29.95,;30.47,-28.41,;31.8,-30.73,;37.35,-26.7,;38.77,-26.1,)| Show InChI InChI=1S/C28H25ClN8O2/c1-34-15-20(11-30)32-26(34)24-23-25(33-37(24)14-18-8-9-31-22-7-6-19(29)10-21(18)22)35(12-16-2-3-16)28(39)36(27(23)38)13-17-4-5-17/h6-10,15-17H,2-5,12-14H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421076
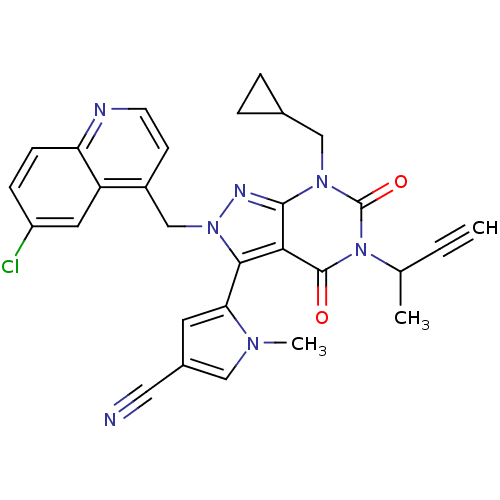
(CHEMBL2087867)Show SMILES CC(C#C)n1c(=O)n(CC2CC2)c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3cc(cn3C)C#N)c2c1=O |(29.34,1.4,;29.35,-.14,;28.02,-.91,;26.68,-1.67,;30.69,-.9,;30.69,-2.44,;29.35,-3.21,;32.01,-3.2,;32.01,-4.74,;30.68,-5.51,;29.14,-5.52,;29.91,-6.86,;33.34,-2.44,;34.81,-2.92,;35.72,-1.67,;37.26,-1.67,;38.03,-3.01,;37.25,-4.34,;38.02,-5.67,;39.57,-5.67,;40.33,-4.32,;41.86,-4.32,;42.62,-2.99,;41.84,-1.66,;42.6,-.32,;40.31,-1.68,;39.56,-3,;34.81,-.43,;35.61,.89,;37.14,1.02,;37.49,2.52,;36.17,3.32,;35,2.31,;33.5,2.65,;38.9,3.12,;40.32,3.73,;33.34,-.9,;32.01,-.12,;32.01,1.42,)| Show InChI InChI=1S/C29H24ClN7O2/c1-4-17(2)37-28(38)25-26(24-11-19(13-31)14-34(24)3)36(33-27(25)35(29(37)39)15-18-5-6-18)16-20-9-10-32-23-8-7-21(30)12-22(20)23/h1,7-12,14,17-18H,5-6,15-16H2,2-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421046

(CHEMBL2087624)Show SMILES CC(C)Cn1c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3cncn3C)c2c(=O)n(C)c1=O |(10.21,-35.14,;10.21,-33.6,;8.87,-32.83,;11.54,-32.83,;11.54,-31.29,;12.87,-30.53,;14.34,-31.01,;15.24,-29.76,;16.78,-29.76,;17.55,-31.09,;16.78,-32.42,;17.55,-33.76,;19.09,-33.76,;19.86,-32.41,;21.39,-32.4,;22.15,-31.08,;21.37,-29.75,;22.13,-28.41,;19.84,-29.76,;19.09,-31.09,;14.34,-28.51,;15.13,-27.2,;16.67,-27.06,;17.02,-25.56,;15.69,-24.77,;14.53,-25.78,;13.03,-25.43,;12.87,-28.99,;11.54,-28.21,;11.54,-26.67,;10.21,-28.99,;8.88,-28.22,;10.21,-30.53,;8.88,-31.3,)| Show InChI InChI=1S/C24H24ClN7O2/c1-14(2)11-31-22-20(23(33)30(4)24(31)34)21(19-10-26-13-29(19)3)32(28-22)12-15-7-8-27-18-6-5-16(25)9-17(15)18/h5-10,13-14H,11-12H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
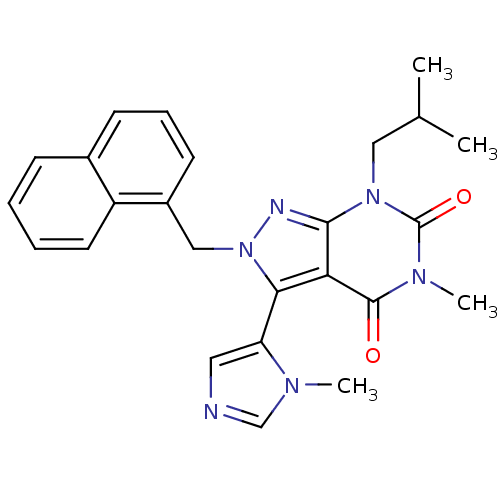
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421061
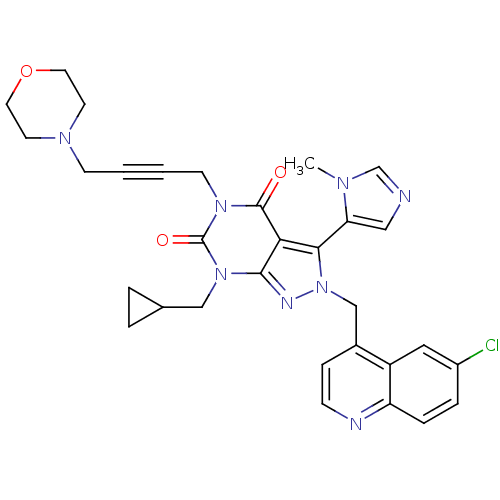
(CHEMBL2087852)Show SMILES Cn1cncc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(CC#CCN3CCOCC3)c(=O)c12 |(33.19,-42.5,;34.69,-42.85,;35.85,-41.84,;37.17,-42.63,;36.83,-44.13,;35.29,-44.27,;34.5,-45.58,;35.4,-46.83,;36.94,-46.83,;37.71,-48.16,;36.94,-49.49,;37.71,-50.83,;39.25,-50.83,;40.02,-49.48,;41.54,-49.47,;42.3,-48.15,;41.53,-46.82,;42.28,-45.48,;40,-46.83,;39.25,-48.16,;34.5,-48.08,;33.03,-47.6,;31.7,-48.36,;31.7,-49.9,;30.37,-50.67,;28.83,-50.68,;29.6,-52.01,;30.37,-47.6,;29.04,-48.37,;30.37,-46.06,;29.04,-45.29,;29.01,-43.75,;29.02,-42.21,;29.03,-40.67,;30.36,-39.91,;31.68,-40.69,;33.02,-39.93,;33.03,-38.39,;31.7,-37.61,;30.36,-38.37,;31.7,-45.28,;31.7,-43.74,;33.03,-46.06,)| Show InChI InChI=1S/C31H31ClN8O3/c1-36-20-33-17-26(36)28-27-29(35-40(28)19-22-8-9-34-25-7-6-23(32)16-24(22)25)39(18-21-4-5-21)31(42)38(30(27)41)11-3-2-10-37-12-14-43-15-13-37/h6-9,16-17,20-21H,4-5,10-15,18-19H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421082
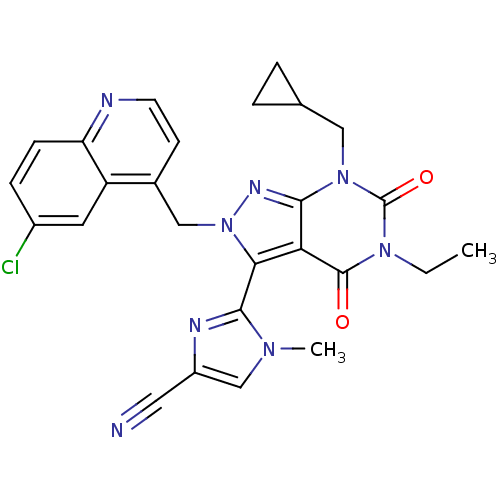
(CHEMBL2088117)Show SMILES CCn1c(=O)n(CC2CC2)c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3nc(cn3C)C#N)c2c1=O |(-8.79,-41.77,;-8.79,-43.31,;-7.45,-44.08,;-7.45,-45.62,;-8.78,-46.39,;-6.12,-46.38,;-6.12,-47.92,;-7.46,-48.69,;-9,-48.7,;-8.23,-50.03,;-4.79,-45.62,;-3.33,-46.1,;-2.42,-44.85,;-.88,-44.85,;-.11,-46.19,;-.88,-47.51,;-.11,-48.85,;1.43,-48.85,;2.19,-47.5,;3.72,-47.49,;4.48,-46.17,;3.7,-44.84,;4.46,-43.5,;2.18,-44.86,;1.43,-46.18,;-3.33,-43.6,;-2.53,-42.29,;-1,-42.15,;-.65,-40.65,;-1.97,-39.86,;-3.13,-40.87,;-4.63,-40.52,;.76,-40.05,;2.18,-39.45,;-4.79,-44.08,;-6.12,-43.3,;-6.12,-41.76,)| Show InChI InChI=1S/C26H23ClN8O2/c1-3-33-25(36)21-22(24-30-18(11-28)14-32(24)2)35(31-23(21)34(26(33)37)12-15-4-5-15)13-16-8-9-29-20-7-6-17(27)10-19(16)20/h6-10,14-15H,3-5,12-13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339998

((R)-2-(3-amino-1-phenylpropylthio)-4-chlorobenzoni...)Show InChI InChI=1S/C16H15ClN2S/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421062
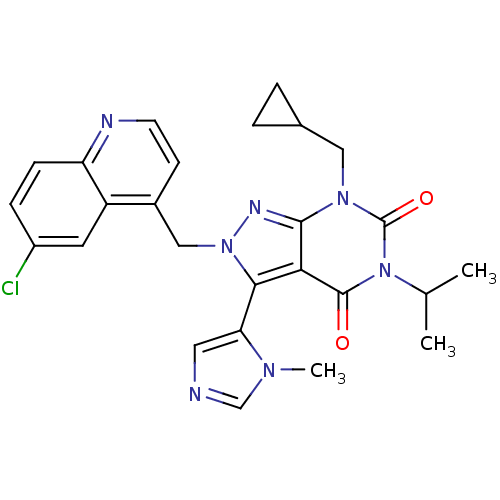
(CHEMBL2087853)Show SMILES CC(C)n1c(=O)n(CC2CC2)c2nn(Cc3ccnc4ccc(Cl)cc34)c(-c3cncn3C)c2c1=O |(-7.2,2.23,;-7.19,.69,;-8.53,-.09,;-5.86,-.08,;-5.86,-1.62,;-7.19,-2.39,;-4.53,-2.38,;-4.53,-3.92,;-5.86,-4.69,;-7.4,-4.7,;-6.63,-6.03,;-3.2,-1.62,;-1.73,-2.1,;-.83,-.85,;.71,-.85,;1.48,-2.18,;.71,-3.51,;1.48,-4.85,;3.02,-4.85,;3.79,-3.5,;5.32,-3.49,;6.08,-2.16,;5.3,-.84,;6.06,.5,;3.77,-.85,;3.02,-2.18,;-1.73,.4,;-.94,1.71,;.6,1.85,;.95,3.35,;-.38,4.14,;-1.54,3.13,;-3.04,3.48,;-3.2,-.08,;-4.53,.7,;-4.53,2.24,)| Show InChI InChI=1S/C26H26ClN7O2/c1-15(2)34-25(35)22-23(21-11-28-14-31(21)3)33(30-24(22)32(26(34)36)12-16-4-5-16)13-17-8-9-29-20-7-6-18(27)10-19(17)20/h6-11,14-16H,4-5,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421047
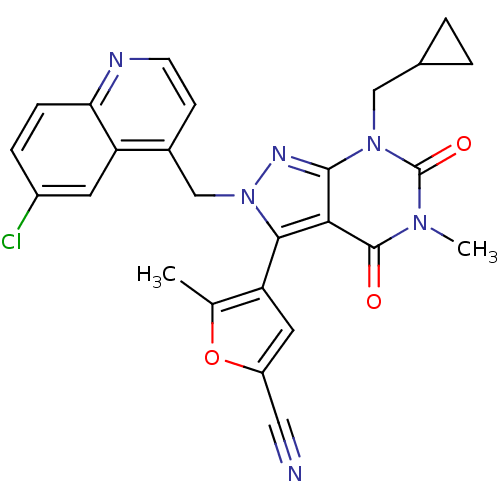
(CHEMBL2087625)Show SMILES Cc1oc(cc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12)C#N |(31.11,-26.6,;32.61,-26.95,;33.77,-25.94,;35.09,-26.73,;34.75,-28.23,;33.21,-28.37,;32.41,-29.68,;33.32,-30.93,;34.86,-30.93,;35.63,-32.26,;34.86,-33.59,;35.63,-34.92,;37.17,-34.93,;37.94,-33.58,;39.46,-33.57,;40.22,-32.24,;39.45,-30.92,;40.2,-29.58,;37.92,-30.93,;37.17,-32.26,;32.42,-32.18,;30.95,-31.7,;29.62,-32.46,;29.62,-34,;28.29,-34.77,;26.75,-34.78,;27.52,-36.11,;28.29,-31.7,;26.96,-32.47,;28.29,-30.16,;26.95,-29.39,;29.62,-29.38,;29.62,-27.84,;30.95,-30.16,;36.5,-26.13,;37.92,-25.53,)| Show InChI InChI=1S/C26H21ClN6O3/c1-14-19(10-18(11-28)36-14)23-22-24(32(12-15-3-4-15)26(35)31(2)25(22)34)30-33(23)13-16-7-8-29-21-6-5-17(27)9-20(16)21/h5-10,15H,3-4,12-13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421048
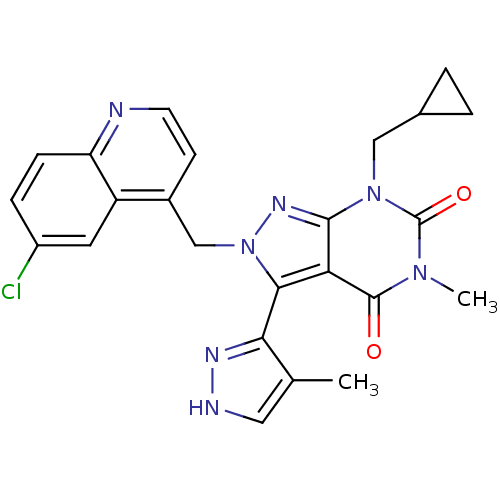
(CHEMBL2087626)Show SMILES Cc1c[nH]nc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12 |(-4.59,-40.17,;-3.09,-40.52,;-1.93,-39.51,;-.61,-40.3,;-.96,-41.8,;-2.49,-41.94,;-3.29,-43.25,;-2.38,-44.5,;-.84,-44.5,;-.07,-45.83,;-.84,-47.16,;-.07,-48.49,;1.47,-48.49,;2.23,-47.15,;3.76,-47.14,;4.52,-45.81,;3.74,-44.49,;4.5,-43.15,;2.22,-44.5,;1.46,-45.82,;-3.29,-45.75,;-4.75,-45.27,;-6.08,-46.03,;-6.08,-47.57,;-7.42,-48.34,;-8.96,-48.35,;-8.19,-49.68,;-7.41,-45.27,;-8.74,-46.04,;-7.41,-43.73,;-8.75,-42.96,;-6.08,-42.95,;-6.08,-41.41,;-4.75,-43.73,)| Show InChI InChI=1S/C24H22ClN7O2/c1-13-10-27-28-20(13)21-19-22(31(11-14-3-4-14)24(34)30(2)23(19)33)29-32(21)12-15-7-8-26-18-6-5-16(25)9-17(15)18/h5-10,14H,3-4,11-12H2,1-2H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |
Glutamate racemase
(Helicobacter pylori J99) | BDBM50421049
(CHEMBL2087627)Show SMILES Cn1cnnc1-c1n(Cc2ccnc3ccc(Cl)cc23)nc2n(CC3CC3)c(=O)n(C)c(=O)c12 |(13.36,-40.21,;14.86,-40.56,;16.02,-39.55,;17.35,-40.34,;17,-41.84,;15.46,-41.98,;14.67,-43.29,;15.57,-44.54,;17.11,-44.54,;17.88,-45.87,;17.11,-47.2,;17.88,-48.54,;19.42,-48.54,;20.19,-47.19,;21.72,-47.18,;22.48,-45.86,;21.7,-44.53,;22.46,-43.19,;20.17,-44.54,;19.42,-45.87,;14.67,-45.79,;13.2,-45.31,;11.87,-46.07,;11.87,-47.61,;10.54,-48.38,;9,-48.39,;9.77,-49.72,;10.54,-45.31,;9.21,-46.08,;10.54,-43.77,;9.21,-43,;11.87,-42.99,;11.87,-41.45,;13.2,-43.77,)| Show InChI InChI=1S/C23H21ClN8O2/c1-29-12-26-27-21(29)19-18-20(31(10-13-3-4-13)23(34)30(2)22(18)33)28-32(19)11-14-7-8-25-17-6-5-15(24)9-16(14)17/h5-9,12-13H,3-4,10-11H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori J99 glutamate racemase |
Bioorg Med Chem Lett 22: 5600-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.004
BindingDB Entry DOI: 10.7270/Q2NZ88XM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data