| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50430655 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_942092 (CHEMBL2340790) |
|---|
| IC50 | 17820±n/a nM |
|---|
| Citation |  Kia, Y; Osman, H; Kumar, RS; Murugaiyah, V; Basiri, A; Perumal, S; Wahab, HA; Bing, CS Synthesis and discovery of novel piperidone-grafted mono- and bis-spirooxindole-hexahydropyrrolizines as potent cholinesterase inhibitors. Bioorg Med Chem21:1696-707 (2013) [PubMed] Article Kia, Y; Osman, H; Kumar, RS; Murugaiyah, V; Basiri, A; Perumal, S; Wahab, HA; Bing, CS Synthesis and discovery of novel piperidone-grafted mono- and bis-spirooxindole-hexahydropyrrolizines as potent cholinesterase inhibitors. Bioorg Med Chem21:1696-707 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | BCHE | Butyrylcholinesterase (BuChE) | CHLE_HORSE | Cholinesterase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 65643.35 |
|---|
| Organism: | Equus caballus (Horse) |
|---|
| Description: | P81908 |
|---|
| Residue: | 574 |
|---|
| Sequence: | EEDIIITTKNGKVRGMNLPVLGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSNIWNATK
YANSCYQNTDQSFPGFLGSEMWNPNTELSEDCLYLNVWIPAPKPKNATVMIWIYGGGFQT
GTSSLPVYDGKFLARVERVIVVSMNYRVGALGFLALSENPEAPGNMGLFDQQLALQWVQK
NIAAFGGNPRSVTLFGESAGAASVSLHLLSPRSQPLFTRAILQSGSSNAPWAVTSLYEAR
NRTLTLAKRMGCSRDNETEMIKCLRDKDPQEILLNEVFVVPYDTLLSVNFGPTVDGDFLT
DMPDTLLQLGQFKRTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPR
VSEFGRESILFHYMDWLDDQRAENYREALDDVVGDYNIICPALEFTRKFSELGNDAFFYY
FEHRSTKLPWPEWMGVMHGYEIEFVFGLPLERRVNYTRAEEILSRSIMKRWANFAKYGNP
NGTQNNSTRWPVFKSTEQKYLTLNTESPKVYTKLRAQQCRFWTLFFPKVLELTGNIDEAE
REWKAGFHRWNNYMMDWKNQFNDYTSKKESCSDF
|
|
|
|---|
| BDBM50430655 |
|---|
| n/a |
|---|
| Name | BDBM50430655 |
|---|
| Synonyms: | CHEMBL2332549 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C37H37N3O3 |
|---|
| Mol. Mass. | 571.708 |
|---|
| SMILES | Cc1ccccc1\C=C1/CN(C\C(=C/c2ccccc2C)C1=O)C(=O)C[C@H]1C[C@H]2CCCN2[C@]11C(=O)Nc2ccccc12 |r| |
|---|
| Structure | 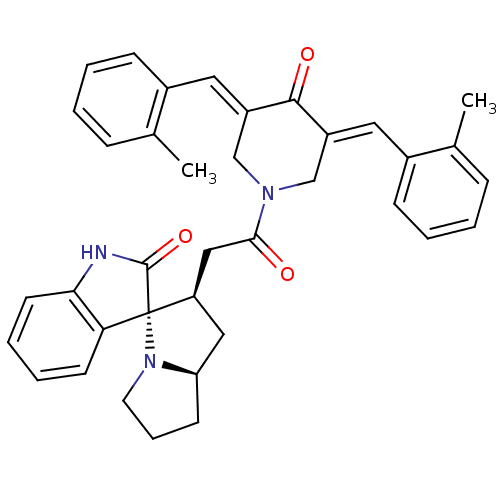
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kia, Y; Osman, H; Kumar, RS; Murugaiyah, V; Basiri, A; Perumal, S; Wahab, HA; Bing, CS Synthesis and discovery of novel piperidone-grafted mono- and bis-spirooxindole-hexahydropyrrolizines as potent cholinesterase inhibitors. Bioorg Med Chem21:1696-707 (2013) [PubMed] Article
Kia, Y; Osman, H; Kumar, RS; Murugaiyah, V; Basiri, A; Perumal, S; Wahab, HA; Bing, CS Synthesis and discovery of novel piperidone-grafted mono- and bis-spirooxindole-hexahydropyrrolizines as potent cholinesterase inhibitors. Bioorg Med Chem21:1696-707 (2013) [PubMed] Article