

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50447970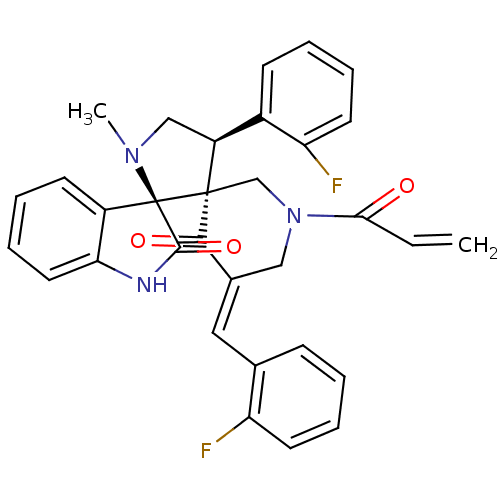 (CHEMBL3115043) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate by Dixon plot analysis | Bioorg Med Chem 22: 1318-28 (2014) Article DOI: 10.1016/j.bmc.2014.01.002 BindingDB Entry DOI: 10.7270/Q25X2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50445716 (CHEMBL3104317) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206892 (CHEMBL3948489) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50434513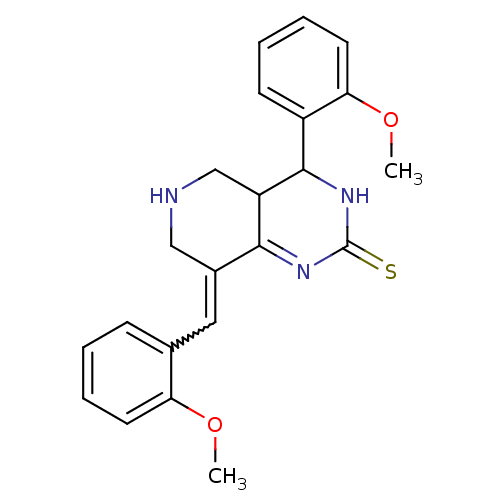 (CHEMBL2385778) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by E... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50445693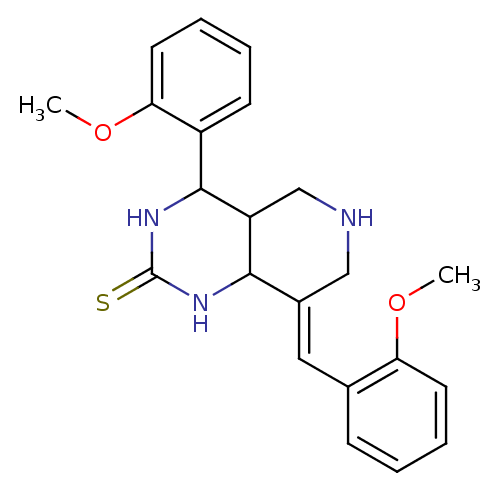 (CHEMBL3104448) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206895 (CHEMBL3983017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50439104 (CHEMBL2417659) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured after 30... | Eur J Med Chem 67: 221-9 (2013) Article DOI: 10.1016/j.ejmech.2013.06.054 BindingDB Entry DOI: 10.7270/Q2VH5Q78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206890 (CHEMBL3910830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206891 (CHEMBL3921785) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206897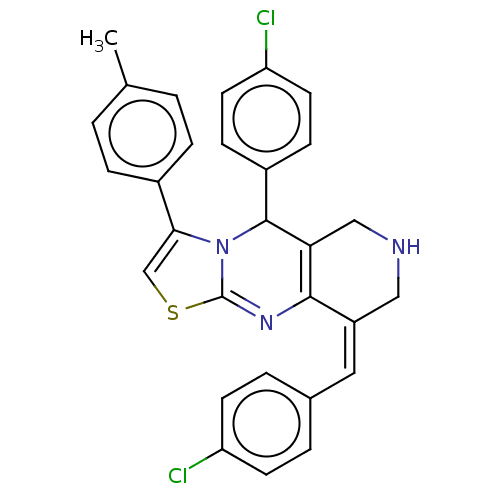 (CHEMBL3945423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50445693 (CHEMBL3104448) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50434513 (CHEMBL2385778) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50439097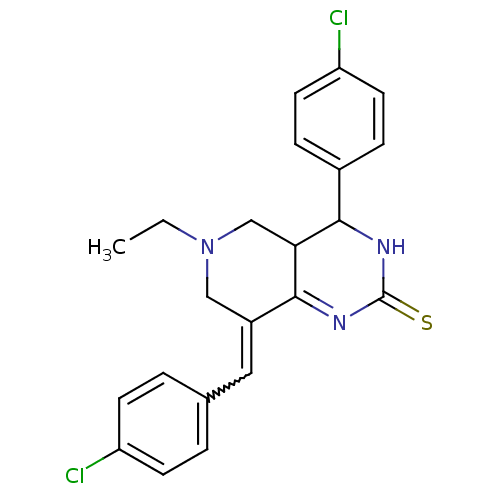 (CHEMBL1987383) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured after 30... | Eur J Med Chem 67: 221-9 (2013) Article DOI: 10.1016/j.ejmech.2013.06.054 BindingDB Entry DOI: 10.7270/Q2VH5Q78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206885 (CHEMBL3930722) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50434506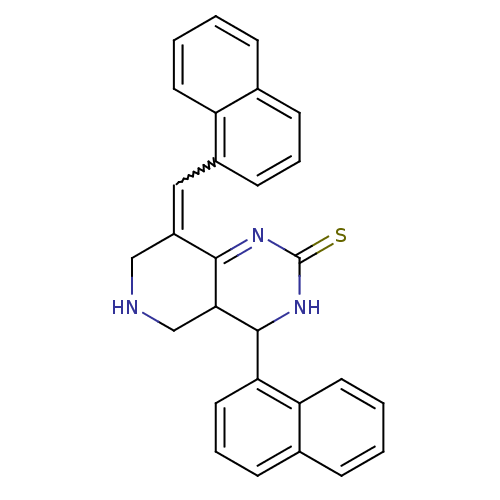 (CHEMBL2385785) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by E... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50445719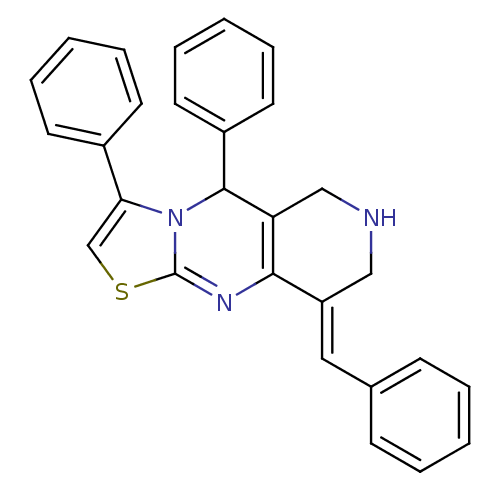 (CHEMBL3104452) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206896 (CHEMBL3958208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206889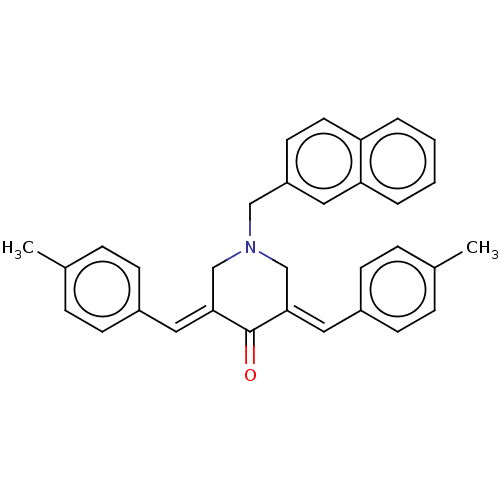 (CHEMBL3928840) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50445715 (CHEMBL3104318) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50434511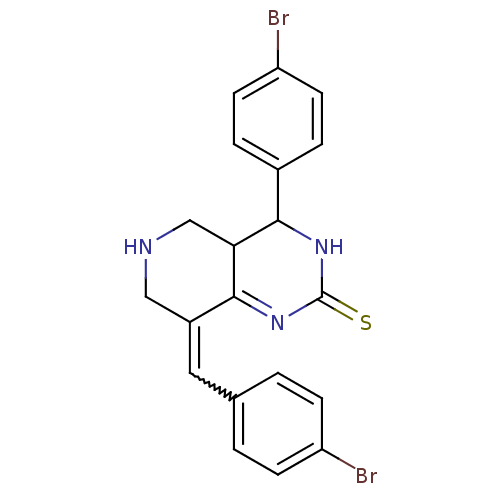 (CHEMBL2385780) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50008529 (CHEMBL3235940) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins by spectrophotometer analysis | Bioorg Med Chem Lett 24: 1815-9 (2014) Article DOI: 10.1016/j.bmcl.2014.02.019 BindingDB Entry DOI: 10.7270/Q2S46TH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50445716 (CHEMBL3104317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50445719 (CHEMBL3104452) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206893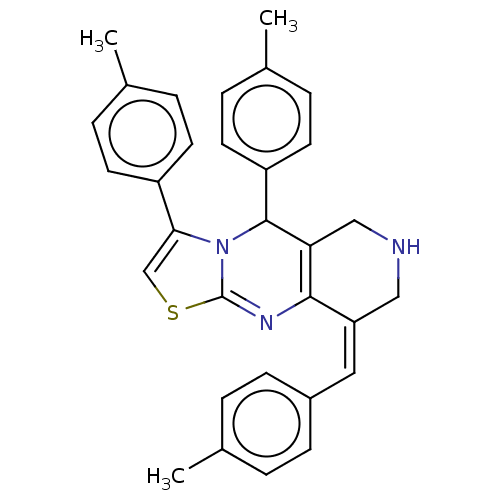 (CHEMBL3901794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50439093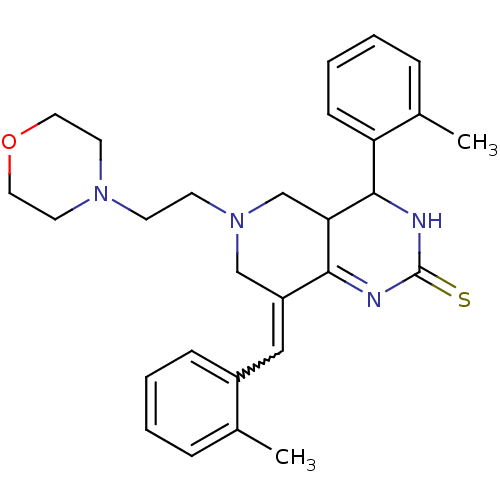 (CHEMBL2417668) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition measured afte... | Eur J Med Chem 67: 221-9 (2013) Article DOI: 10.1016/j.ejmech.2013.06.054 BindingDB Entry DOI: 10.7270/Q2VH5Q78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206886 (CHEMBL3955393) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50445718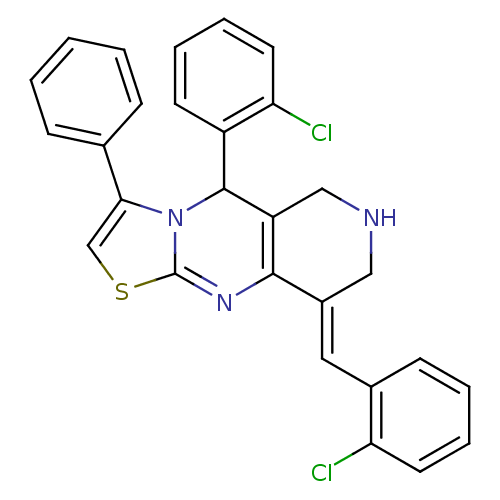 (CHEMBL3104453) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50439096 (CHEMBL2417665) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured after 30... | Eur J Med Chem 67: 221-9 (2013) Article DOI: 10.1016/j.ejmech.2013.06.054 BindingDB Entry DOI: 10.7270/Q2VH5Q78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50008675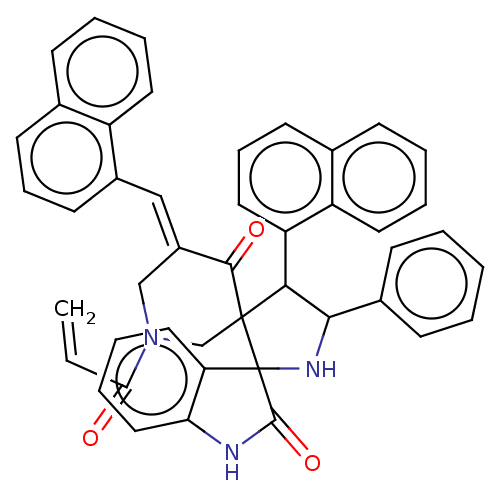 (CHEMBL3235947) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins by spectrophotometer analysis | Bioorg Med Chem Lett 24: 1815-9 (2014) Article DOI: 10.1016/j.bmcl.2014.02.019 BindingDB Entry DOI: 10.7270/Q2S46TH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins by spectrophotometer analysis | Bioorg Med Chem Lett 24: 1815-9 (2014) Article DOI: 10.1016/j.bmcl.2014.02.019 BindingDB Entry DOI: 10.7270/Q2S46TH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured after 30... | Eur J Med Chem 67: 221-9 (2013) Article DOI: 10.1016/j.ejmech.2013.06.054 BindingDB Entry DOI: 10.7270/Q2VH5Q78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by E... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50445715 (CHEMBL3104318) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50434509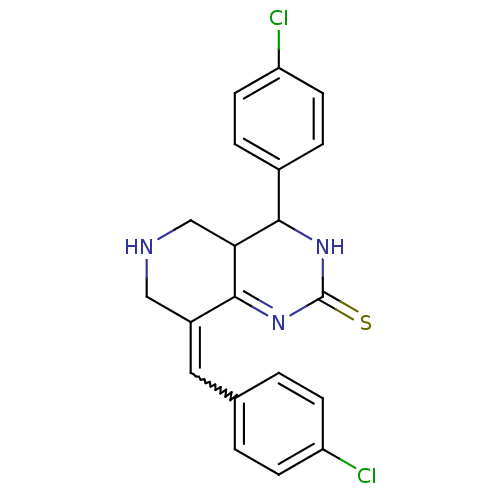 (CHEMBL2385782) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine iodide as substrate incubated for 15 mins prior to substrate addition measured after 30 mins by E... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50445721 (CHEMBL3104450) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrat... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50008530 (CHEMBL3235941) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins by spectrophotometer analysis | Bioorg Med Chem Lett 24: 1815-9 (2014) Article DOI: 10.1016/j.bmcl.2014.02.019 BindingDB Entry DOI: 10.7270/Q2S46TH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50206894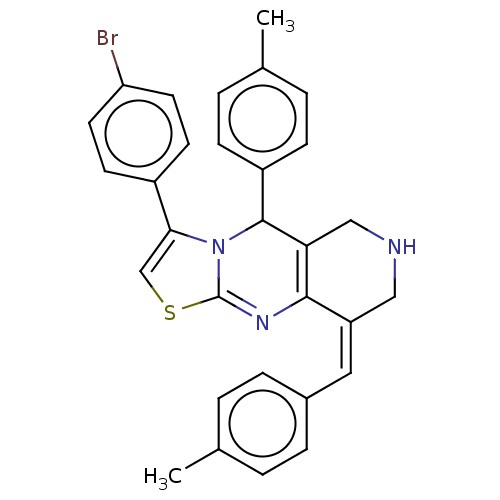 (CHEMBL3972865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc... | Bioorg Med Chem Lett 27: 228-231 (2017) Article DOI: 10.1016/j.bmcl.2016.11.065 BindingDB Entry DOI: 10.7270/Q28054MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50439092 (CHEMBL2417669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition measured afte... | Eur J Med Chem 67: 221-9 (2013) Article DOI: 10.1016/j.ejmech.2013.06.054 BindingDB Entry DOI: 10.7270/Q2VH5Q78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50434527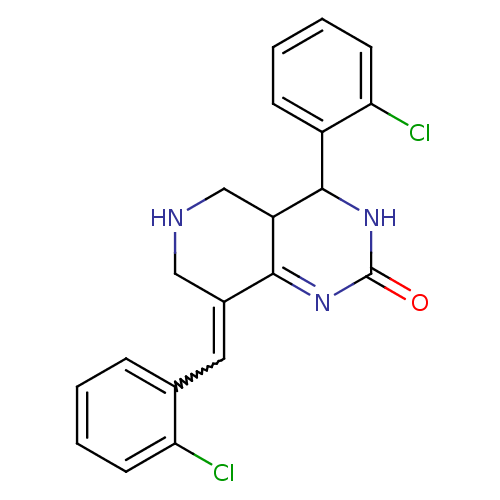 (CHEMBL2385764) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50445718 (CHEMBL3104453) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50008674 (CHEMBL3235946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate preincubated for 15 mins by spectrophotometer analysis | Bioorg Med Chem Lett 24: 1815-9 (2014) Article DOI: 10.1016/j.bmcl.2014.02.019 BindingDB Entry DOI: 10.7270/Q2S46TH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50434529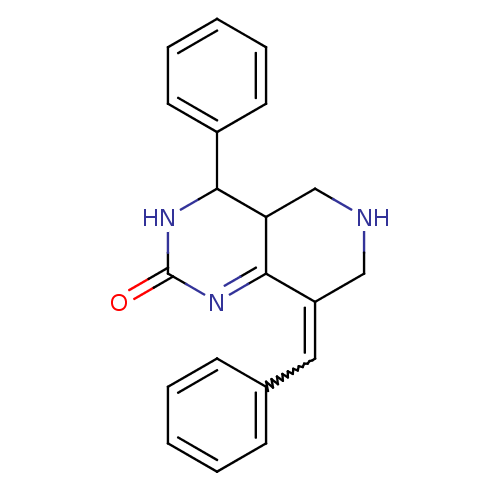 (CHEMBL2385762) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50434515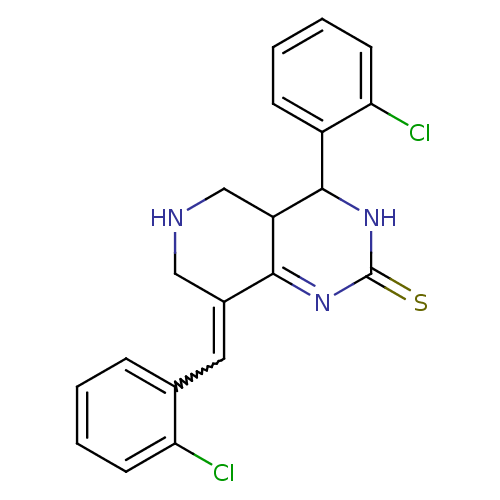 (CHEMBL2385776) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50445694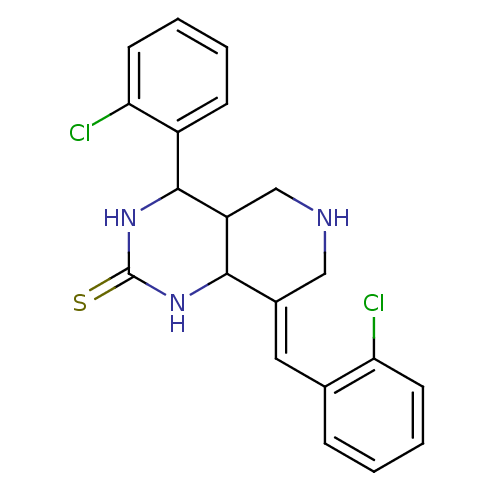 (CHEMBL3104447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addi... | Bioorg Med Chem 22: 906-16 (2014) Article DOI: 10.1016/j.bmc.2013.11.020 BindingDB Entry DOI: 10.7270/Q23R0VBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50439091 (CHEMBL2417670) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using S-butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition measured afte... | Eur J Med Chem 67: 221-9 (2013) Article DOI: 10.1016/j.ejmech.2013.06.054 BindingDB Entry DOI: 10.7270/Q2VH5Q78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50434516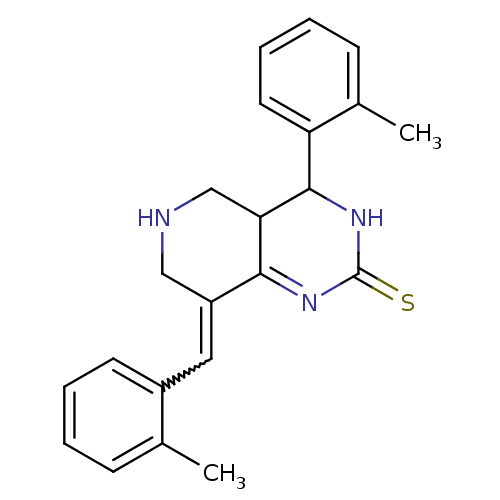 (CHEMBL2385775) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem 21: 3022-31 (2013) Article DOI: 10.1016/j.bmc.2013.03.058 BindingDB Entry DOI: 10.7270/Q2V40WK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433622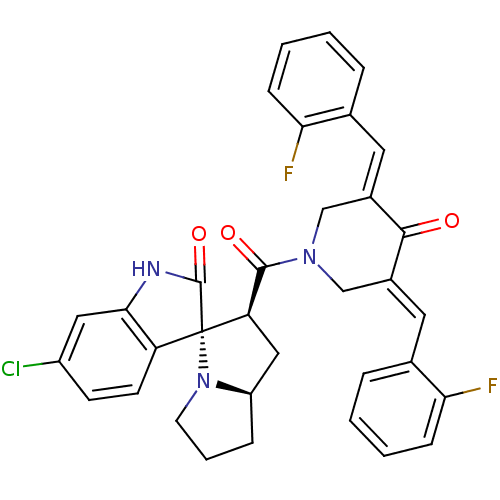 (CHEMBL2380672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem Lett 23: 2979-83 (2013) Article DOI: 10.1016/j.bmcl.2013.03.027 BindingDB Entry DOI: 10.7270/Q2057H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 329 total ) | Next | Last >> |