| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50010462 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1353338 (CHEMBL3268199) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Aoki, T; Hyohdoh, I; Furuichi, N; Ozawa, S; Watanabe, F; Matsushita, M; Sakaitani, M; Morikami, K; Takanashi, K; Harada, N; Tomii, Y; Shiraki, K; Furumoto, K; Tabo, M; Yoshinari, K; Ori, K; Aoki, Y; Shimma, N; Iikura, H Optimizing the Physicochemical Properties of Raf/MEK Inhibitors by Nitrogen Scanning. ACS Med Chem Lett5:309-14 (2014) [PubMed] Article Aoki, T; Hyohdoh, I; Furuichi, N; Ozawa, S; Watanabe, F; Matsushita, M; Sakaitani, M; Morikami, K; Takanashi, K; Harada, N; Tomii, Y; Shiraki, K; Furumoto, K; Tabo, M; Yoshinari, K; Ori, K; Aoki, Y; Shimma, N; Iikura, H Optimizing the Physicochemical Properties of Raf/MEK Inhibitors by Nitrogen Scanning. ACS Med Chem Lett5:309-14 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50010462 |
|---|
| n/a |
|---|
| Name | BDBM50010462 |
|---|
| Synonyms: | CHEBI:78825 | CHEMBL3264002 | US11147816, RO5126766-(CH5126766) | US11701360, R05126766 | US20230270730, Compound ref-5 | US20230382863, Compound avutometinib |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H18FN5O5S |
|---|
| Mol. Mass. | 471.462 |
|---|
| SMILES | CNS(=O)(=O)Nc1nccc(Cc2c(C)c3ccc(Oc4ncccn4)cc3oc2=O)c1F |
|---|
| Structure | 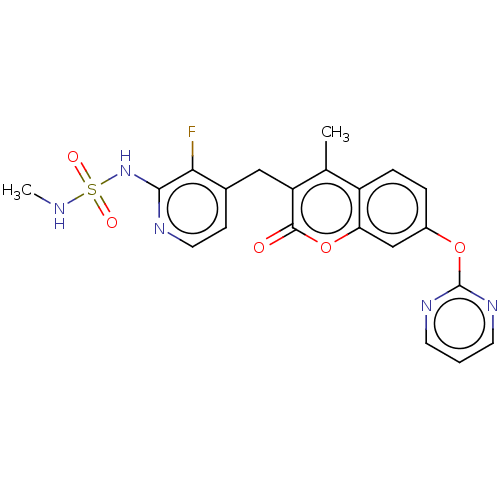
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Aoki, T; Hyohdoh, I; Furuichi, N; Ozawa, S; Watanabe, F; Matsushita, M; Sakaitani, M; Morikami, K; Takanashi, K; Harada, N; Tomii, Y; Shiraki, K; Furumoto, K; Tabo, M; Yoshinari, K; Ori, K; Aoki, Y; Shimma, N; Iikura, H Optimizing the Physicochemical Properties of Raf/MEK Inhibitors by Nitrogen Scanning. ACS Med Chem Lett5:309-14 (2014) [PubMed] Article
Aoki, T; Hyohdoh, I; Furuichi, N; Ozawa, S; Watanabe, F; Matsushita, M; Sakaitani, M; Morikami, K; Takanashi, K; Harada, N; Tomii, Y; Shiraki, K; Furumoto, K; Tabo, M; Yoshinari, K; Ori, K; Aoki, Y; Shimma, N; Iikura, H Optimizing the Physicochemical Properties of Raf/MEK Inhibitors by Nitrogen Scanning. ACS Med Chem Lett5:309-14 (2014) [PubMed] Article