 Found 511 hits with Last Name = 'watanabe' and Initial = 'f'
Found 511 hits with Last Name = 'watanabe' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM8485

((2R)-N-hydroxy-3-methyl-2-[(4-phenoxybenzene)sulfo...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)NO |r| Show InChI InChI=1S/C17H20N2O5S/c1-12(2)16(17(20)18-21)19-25(22,23)15-10-8-14(9-11-15)24-13-6-4-3-5-7-13/h3-12,16,19,21H,1-2H3,(H,18,20)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase B (Matrix metalloproteinase-9) |
J Med Chem 41: 640-9 (1998)
Article DOI: 10.1021/jm9707582
BindingDB Entry DOI: 10.7270/Q2HD7TS9 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50077162

((R)-3-(1H-Indol-3-yl)-2-[4-(4-methoxy-phenylethyny...)Show SMILES COc1ccc(cc1)C#Cc1ccc(cc1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C26H22N2O5S/c1-33-21-12-8-18(9-13-21)6-7-19-10-14-22(15-11-19)34(31,32)28-25(26(29)30)16-20-17-27-24-5-3-2-4-23(20)24/h2-5,8-15,17,25,27-28H,16H2,1H3,(H,29,30)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) |
J Med Chem 42: 1723-38 (1999)
Article DOI: 10.1021/jm980514x
BindingDB Entry DOI: 10.7270/Q2KP81BQ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593047
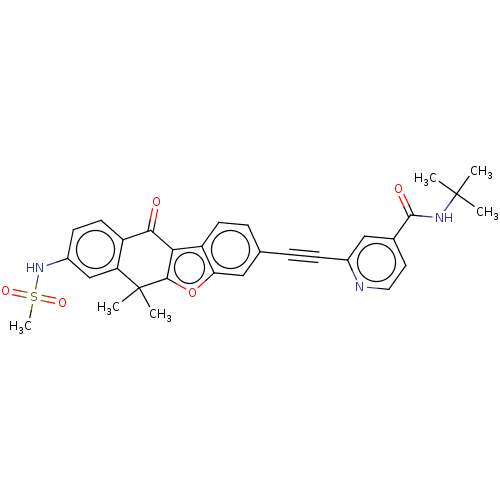
(CHEMBL5172448)Show SMILES CC(C)(C)NC(=O)c1ccnc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593050
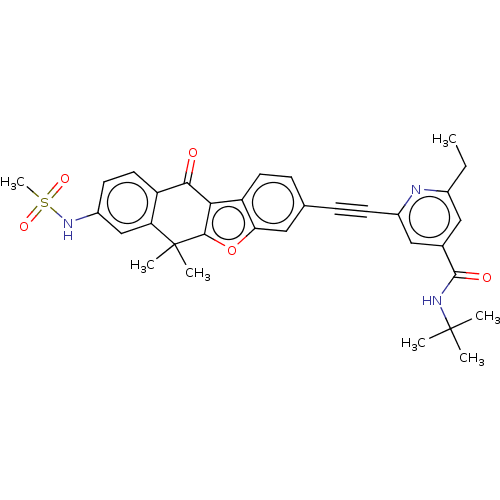
(CHEMBL5174525)Show SMILES CCc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in EGF-stimulated human A431 after 2 hrs by Western blot analysis |
Bioorg Med Chem Lett 22: 456-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.103
BindingDB Entry DOI: 10.7270/Q2NV9JP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of EGFR by homogeneous time-resolved fluorescence assay |
Bioorg Med Chem Lett 21: 1601-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.119
BindingDB Entry DOI: 10.7270/Q2RX9CCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of EGFR by homogeneous time-resolved fluorescence assay |
Bioorg Med Chem Lett 21: 1601-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.119
BindingDB Entry DOI: 10.7270/Q2RX9CCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in EGF-stimulated human A431 after 2 hrs by Western blot analysis |
Bioorg Med Chem Lett 22: 456-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.103
BindingDB Entry DOI: 10.7270/Q2NV9JP4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593051
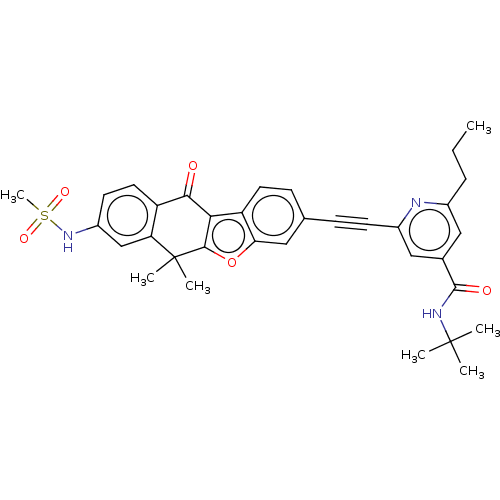
(CHEMBL5171942)Show SMILES CCCc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063148
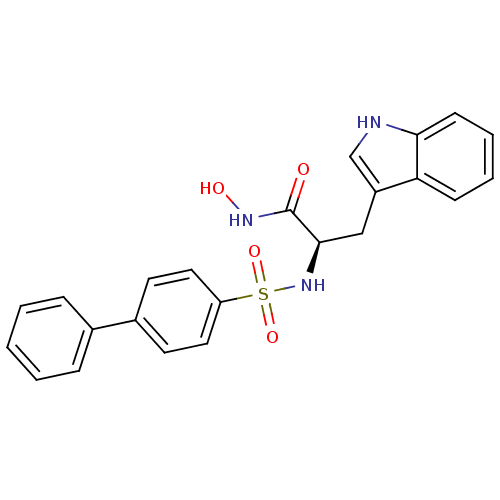
((R)-2-(Biphenyl-4-sulfonylamino)-N-hydroxy-3-(1H-i...)Show SMILES ONC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C23H21N3O4S/c27-23(25-28)22(14-18-15-24-21-9-5-4-8-20(18)21)26-31(29,30)19-12-10-17(11-13-19)16-6-2-1-3-7-16/h1-13,15,22,24,26,28H,14H2,(H,25,27)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase A (matrix metalloproteinase-2 MMP2) |
J Med Chem 41: 640-9 (1998)
Article DOI: 10.1021/jm9707582
BindingDB Entry DOI: 10.7270/Q2HD7TS9 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593035

(CHEMBL5183149)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(ccn1)C(=O)NC1CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593043
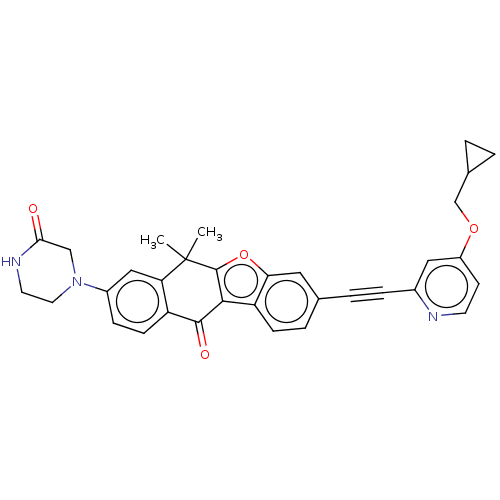
(CHEMBL5189763)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CCNC(=O)C1)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50077157

((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...)Show SMILES CCc1ccc(cc1)C#Cc1ccc(s1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C25H22N2O4S2/c1-2-17-7-9-18(10-8-17)11-12-20-13-14-24(32-20)33(30,31)27-23(25(28)29)15-19-16-26-22-6-4-3-5-21(19)22/h3-10,13-14,16,23,26-27H,2,15H2,1H3,(H,28,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) |
J Med Chem 42: 1723-38 (1999)
Article DOI: 10.1021/jm980514x
BindingDB Entry DOI: 10.7270/Q2KP81BQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063129
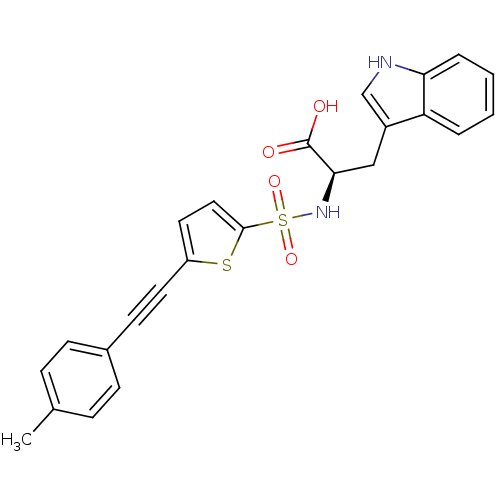
((R)-3-(1H-Indol-3-yl)-2-(5-p-tolylethynyl-thiophen...)Show SMILES Cc1ccc(cc1)C#Cc1ccc(s1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C24H20N2O4S2/c1-16-6-8-17(9-7-16)10-11-19-12-13-23(31-19)32(29,30)26-22(24(27)28)14-18-15-25-21-5-3-2-4-20(18)21/h2-9,12-13,15,22,25-26H,14H2,1H3,(H,27,28)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) |
J Med Chem 42: 1723-38 (1999)
Article DOI: 10.1021/jm980514x
BindingDB Entry DOI: 10.7270/Q2KP81BQ |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50085993
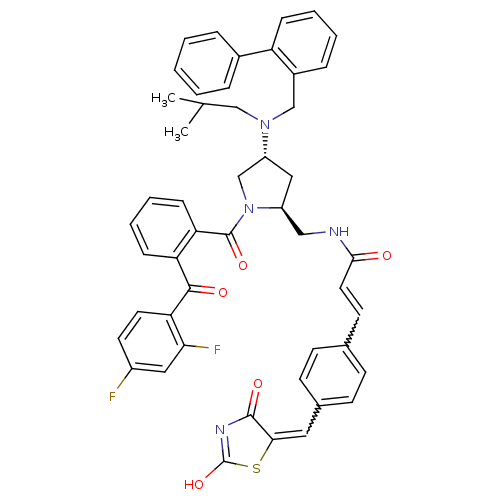
((E)-N-(((2S,4R)-4-((biphenyl-2-ylmethyl)(isobutyl)...)Show SMILES CC(C)CN(Cc1ccccc1-c1ccccc1)[C@@H]1C[C@@H](CNC(=O)C=Cc2ccc(C=C3SC(O)=NC3=O)cc2)N(C1)C(=O)c1ccccc1C(=O)c1ccc(F)cc1F |w:31.32,26.28,c:37| Show InChI InChI=1S/C49H44F2N4O5S/c1-31(2)28-54(29-35-12-6-7-13-39(35)34-10-4-3-5-11-34)38-26-37(27-52-45(56)23-20-32-16-18-33(19-17-32)24-44-47(58)53-49(60)61-44)55(30-38)48(59)41-15-9-8-14-40(41)46(57)42-22-21-36(50)25-43(42)51/h3-25,31,37-38H,26-30H2,1-2H3,(H,52,56)(H,53,58,60)/t37-,38+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) |
J Med Chem 43: 1041-4 (2000)
BindingDB Entry DOI: 10.7270/Q27P8XMZ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM8485

((2R)-N-hydroxy-3-methyl-2-[(4-phenoxybenzene)sulfo...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)NO |r| Show InChI InChI=1S/C17H20N2O5S/c1-12(2)16(17(20)18-21)19-25(22,23)15-10-8-14(9-11-15)24-13-6-4-3-5-7-13/h3-12,16,19,21H,1-2H3,(H,18,20)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase A (matrix metalloproteinase-2 MMP2) |
J Med Chem 41: 640-9 (1998)
Article DOI: 10.1021/jm9707582
BindingDB Entry DOI: 10.7270/Q2HD7TS9 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593031

(CHEMBL5191460)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593029
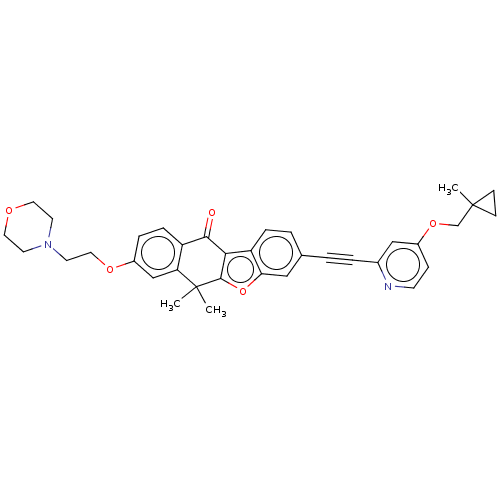
(CHEMBL5195158)Show SMILES CC1(COc2ccnc(c2)C#Cc2ccc3c4c(oc3c2)C(C)(C)c2cc(OCCN3CCOCC3)ccc2C4=O)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50085984
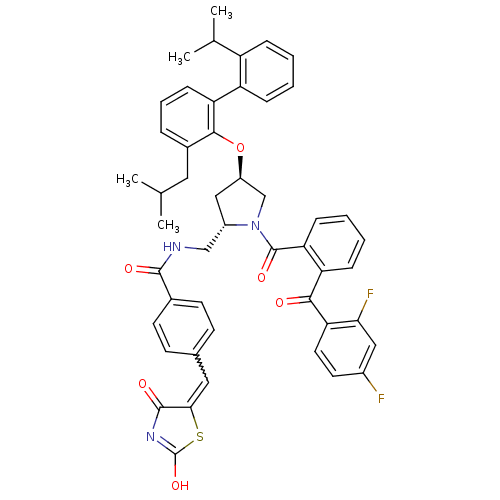
(CHEMBL9161 | N-[1-[2-(2,4-Difluoro-benzoyl)-benzoy...)Show SMILES CC(C)Cc1cccc(c1O[C@@H]1C[C@@H](CNC(=O)c2ccc(C=C3SC(O)=NC3=O)cc2)N(C1)C(=O)c1ccccc1C(=O)c1ccc(F)cc1F)-c1ccccc1C(C)C |w:22.22,c:27| Show InChI InChI=1S/C49H45F2N3O6S/c1-28(2)22-32-10-9-15-39(37-12-6-5-11-36(37)29(3)4)45(32)60-35-25-34(26-52-46(56)31-18-16-30(17-19-31)23-43-47(57)53-49(59)61-43)54(27-35)48(58)40-14-8-7-13-38(40)44(55)41-21-20-33(50)24-42(41)51/h5-21,23-24,28-29,34-35H,22,25-27H2,1-4H3,(H,52,56)(H,53,57,59)/t34-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) |
J Med Chem 43: 1041-4 (2000)
BindingDB Entry DOI: 10.7270/Q27P8XMZ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593039

(CHEMBL5199099)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCNC(=O)C3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593045
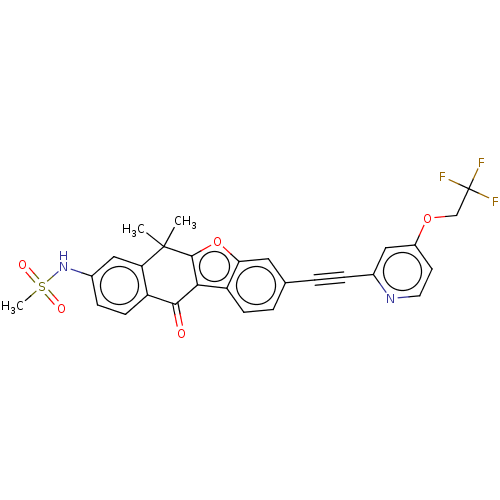
(CHEMBL5205778)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(NS(C)(=O)=O)cc12)C#Cc1cc(OCC(F)(F)F)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593053
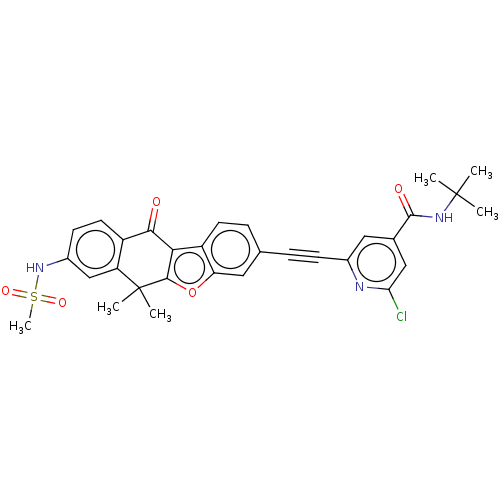
(CHEMBL5198357)Show SMILES CC(C)(C)NC(=O)c1cc(Cl)nc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50020743

(CHEMBL53346 | Sodium; (+)-7-(3-benzenesulfonylamin...)Show SMILES [O-]C(=O)CCC\C=C/C[C@H]1[C@@H]2CCC(C2)[C@@H]1NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H27NO4S/c22-19(23)11-7-2-1-6-10-18-15-12-13-16(14-15)20(18)21-26(24,25)17-8-4-3-5-9-17/h1,3-6,8-9,15-16,18,20-21H,2,7,10-14H2,(H,22,23)/p-1/b6-1-/t15-,16?,18+,20+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of thromboxane A2 synthetase from human platelets by 1 uM of the compound |
J Med Chem 31: 1847-54 (1988)
BindingDB Entry DOI: 10.7270/Q2G44P95 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593046

(CHEMBL5199353)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(NS(C)(=O)=O)cc12)C#Cc1cc(ccn1)C(=O)NC1CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593052
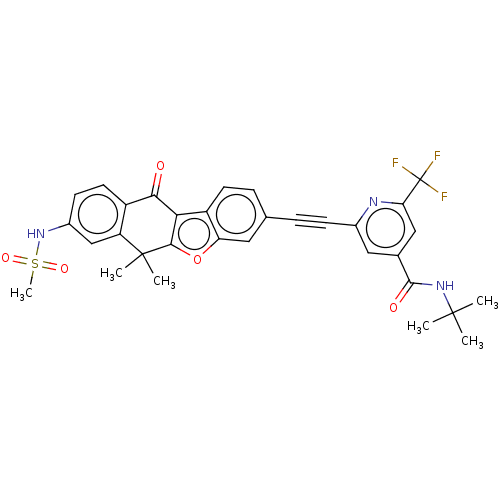
(CHEMBL5178903)Show SMILES CC(C)(C)NC(=O)c1cc(nc(c1)C(F)(F)F)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063139
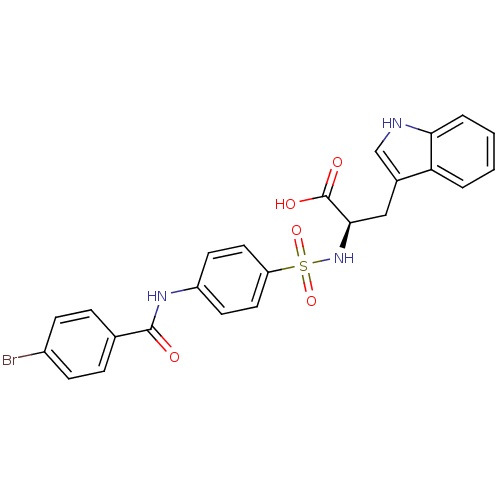
((R)-2-[4-(4-Bromo-benzoylamino)-benzenesulfonylami...)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C24H20BrN3O5S/c25-17-7-5-15(6-8-17)23(29)27-18-9-11-19(12-10-18)34(32,33)28-22(24(30)31)13-16-14-26-21-4-2-1-3-20(16)21/h1-12,14,22,26,28H,13H2,(H,27,29)(H,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase A (matrix metalloproteinase-2 MMP2) |
J Med Chem 41: 640-9 (1998)
Article DOI: 10.1021/jm9707582
BindingDB Entry DOI: 10.7270/Q2HD7TS9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063139

((R)-2-[4-(4-Bromo-benzoylamino)-benzenesulfonylami...)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C24H20BrN3O5S/c25-17-7-5-15(6-8-17)23(29)27-18-9-11-19(12-10-18)34(32,33)28-22(24(30)31)13-16-14-26-21-4-2-1-3-20(16)21/h1-12,14,22,26,28H,13H2,(H,27,29)(H,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase B (Matrix metalloproteinase-9) |
J Med Chem 41: 640-9 (1998)
Article DOI: 10.1021/jm9707582
BindingDB Entry DOI: 10.7270/Q2HD7TS9 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593030
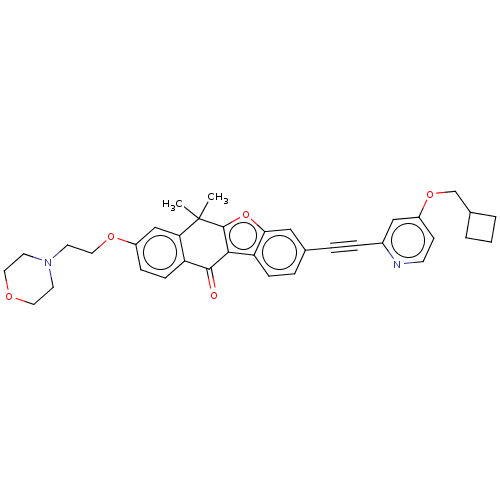
(CHEMBL5193231)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2CCC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593054
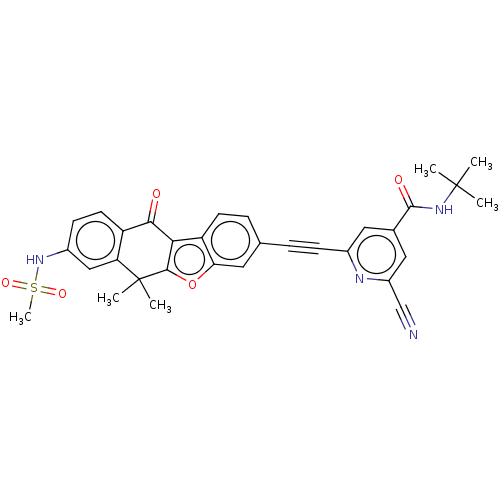
(CHEMBL5173139)Show SMILES CC(C)(C)NC(=O)c1cc(nc(c1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C#N | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593037

(CHEMBL5171199)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCCOCC3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50525707

(CHEMBL4457566)Show SMILES Cc1cc(cc(n1)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C Show InChI InChI=1S/C32H31N3O5S/c1-18-14-20(30(37)34-31(2,3)4)16-21(33-18)10-8-19-9-12-24-26(15-19)40-29-27(24)28(36)23-13-11-22(35-41(7,38)39)17-25(23)32(29,5)6/h9,11-17,35H,1-7H3,(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50338593
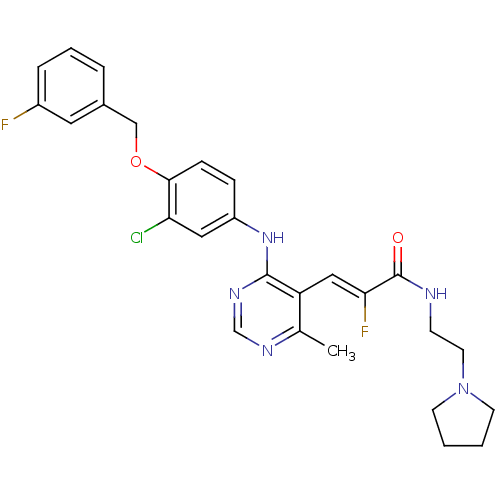
(3-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)-6...)Show SMILES Cc1ncnc(Nc2ccc(OCc3cccc(F)c3)c(Cl)c2)c1\C=C(/F)C(=O)NCCN1CCCC1 Show InChI InChI=1S/C27H28ClF2N5O2/c1-18-22(15-24(30)27(36)31-9-12-35-10-2-3-11-35)26(33-17-32-18)34-21-7-8-25(23(28)14-21)37-16-19-5-4-6-20(29)13-19/h4-8,13-15,17H,2-3,9-12,16H2,1H3,(H,31,36)(H,32,33,34)/b24-15- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of EGFR by homogeneous time-resolved fluorescence assay |
Bioorg Med Chem Lett 21: 1601-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.119
BindingDB Entry DOI: 10.7270/Q2RX9CCN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593042
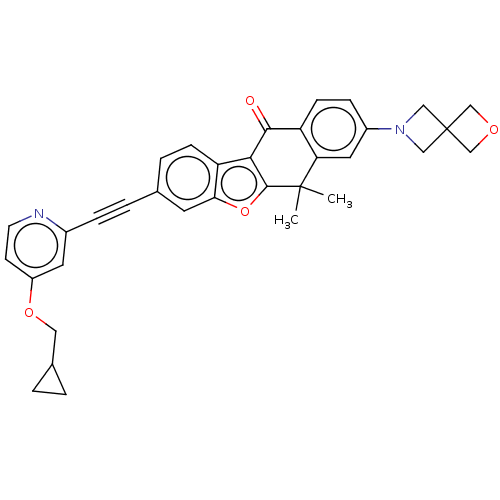
(CHEMBL5169941)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CC2(COC2)C1)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50360457

(CHEMBL1934623)Show SMILES CC(C)NCc1ccc(cc1)C#Cc1c(C)ncnc1Nc1ccc(OCc2cccc(F)c2)c(Cl)c1 Show InChI InChI=1S/C30H28ClFN4O/c1-20(2)33-17-23-9-7-22(8-10-23)11-13-27-21(3)34-19-35-30(27)36-26-12-14-29(28(31)16-26)37-18-24-5-4-6-25(32)15-24/h4-10,12,14-16,19-20,33H,17-18H2,1-3H3,(H,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in EGF-stimulated human A431 after 2 hrs by Western blot analysis |
Bioorg Med Chem Lett 22: 456-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.103
BindingDB Entry DOI: 10.7270/Q2NV9JP4 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50085986

(CHEMBL267258 | N-{4-(Biphenyl-2-ylmethyl-isobutyl-...)Show SMILES CC(C)CN(Cc1ccccc1-c1ccccc1)[C@@H]1C[C@@H](CNC(=O)C=Cc2ccc(C=C3SC(O)=NC3=O)cc2)N(C1)C(=O)c1ccccc1C(=O)c1ccc(F)cc1 |w:31.32,26.28,c:37| Show InChI InChI=1S/C49H45FN4O5S/c1-32(2)29-53(30-37-12-6-7-13-41(37)35-10-4-3-5-11-35)40-27-39(28-51-45(55)25-20-33-16-18-34(19-17-33)26-44-47(57)52-49(59)60-44)54(31-40)48(58)43-15-9-8-14-42(43)46(56)36-21-23-38(50)24-22-36/h3-26,32,39-40H,27-31H2,1-2H3,(H,51,55)(H,52,57,59)/t39-,40+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) |
J Med Chem 43: 1041-4 (2000)
BindingDB Entry DOI: 10.7270/Q27P8XMZ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593033

(CHEMBL5173016)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2(CO)CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593032
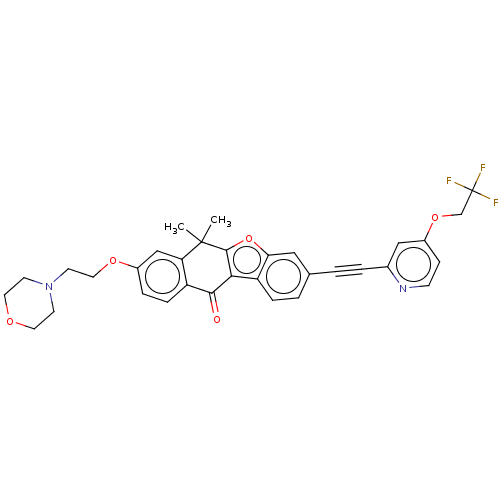
(CHEMBL5171966)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC(F)(F)F)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593041
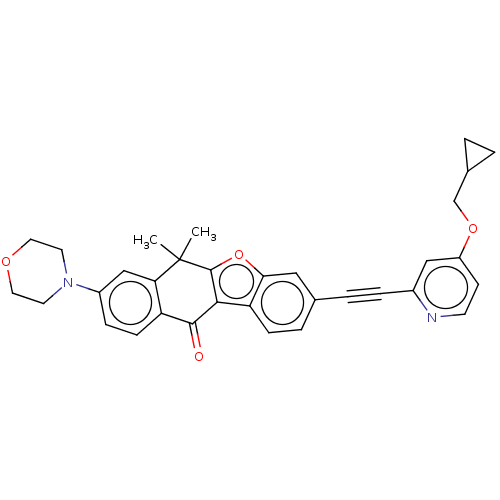
(CHEMBL5198427)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(cc12)N1CCOCC1)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593049
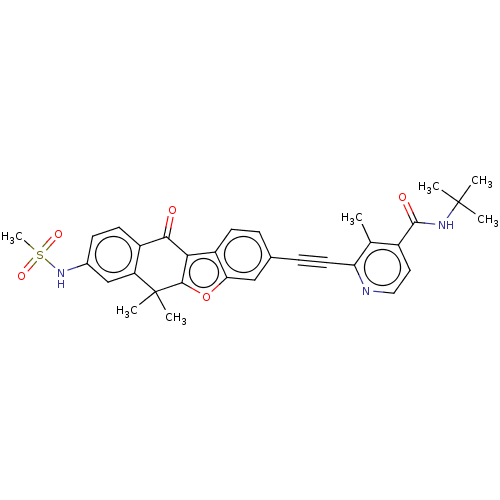
(CHEMBL5203355)Show SMILES Cc1c(ccnc1C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O)C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593048
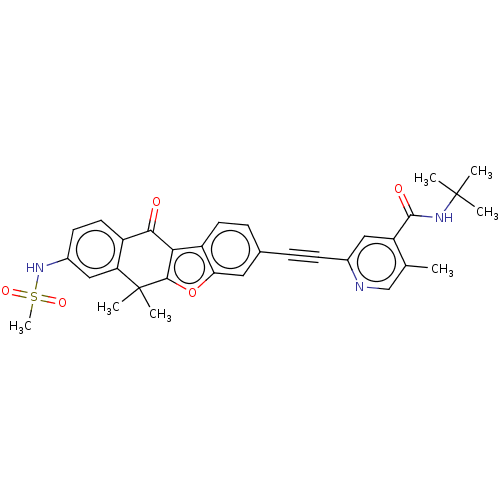
(CHEMBL5174514)Show SMILES Cc1cnc(cc1C(=O)NC(C)(C)C)C#Cc1ccc2c3c(oc2c1)C(C)(C)c1cc(NS(C)(=O)=O)ccc1C3=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593044
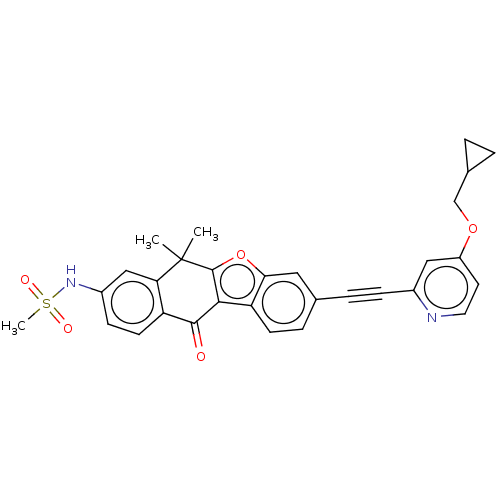
(CHEMBL5199002)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(NS(C)(=O)=O)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50077161
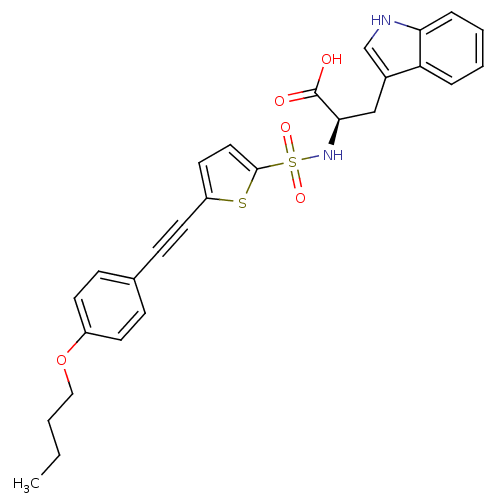
((R)-2-[5-(4-Butoxy-phenylethynyl)-thiophene-2-sulf...)Show SMILES CCCCOc1ccc(cc1)C#Cc1ccc(s1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H26N2O5S2/c1-2-3-16-34-21-11-8-19(9-12-21)10-13-22-14-15-26(35-22)36(32,33)29-25(27(30)31)17-20-18-28-24-7-5-4-6-23(20)24/h4-9,11-12,14-15,18,25,28-29H,2-3,16-17H2,1H3,(H,30,31)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) |
J Med Chem 42: 1723-38 (1999)
Article DOI: 10.1021/jm980514x
BindingDB Entry DOI: 10.7270/Q2KP81BQ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593038
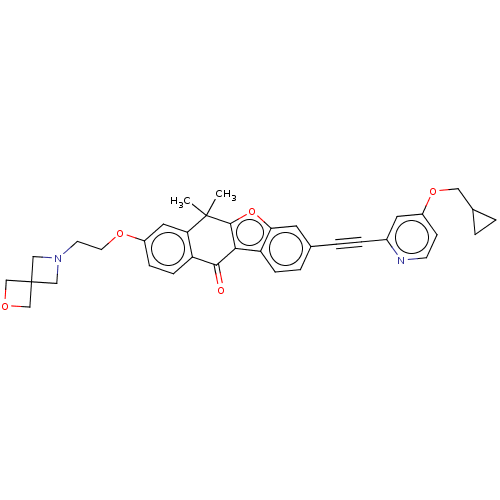
(CHEMBL5197535)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CC4(COC4)C3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063154
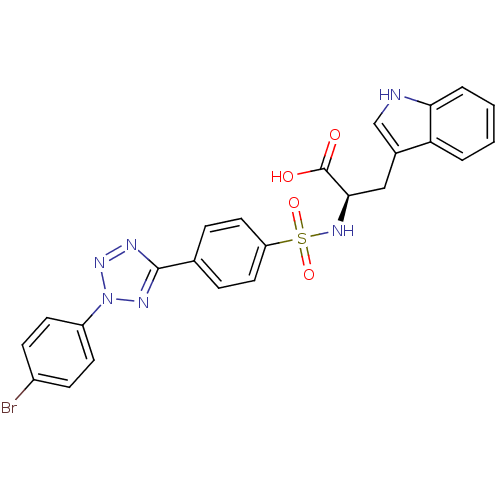
((R)-2-{4-[2-(4-Bromo-phenyl)-2H-tetrazol-5-yl]-ben...)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(cc1)-c1nnn(n1)-c1ccc(Br)cc1 Show InChI InChI=1S/C24H19BrN6O4S/c25-17-7-9-18(10-8-17)31-28-23(27-30-31)15-5-11-19(12-6-15)36(34,35)29-22(24(32)33)13-16-14-26-21-4-2-1-3-20(16)21/h1-12,14,22,26,29H,13H2,(H,32,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase B (Matrix metalloproteinase-9) |
J Med Chem 41: 640-9 (1998)
Article DOI: 10.1021/jm9707582
BindingDB Entry DOI: 10.7270/Q2HD7TS9 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50077152
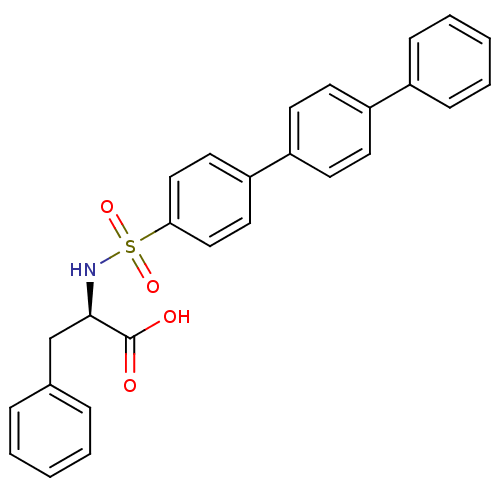
((R)-3-Phenyl-2-([1,1';4',1'']terphenyl-4-sulfonyla...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C27H23NO4S/c29-27(30)26(19-20-7-3-1-4-8-20)28-33(31,32)25-17-15-24(16-18-25)23-13-11-22(12-14-23)21-9-5-2-6-10-21/h1-18,26,28H,19H2,(H,29,30)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) |
J Med Chem 42: 1723-38 (1999)
Article DOI: 10.1021/jm980514x
BindingDB Entry DOI: 10.7270/Q2KP81BQ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50077151
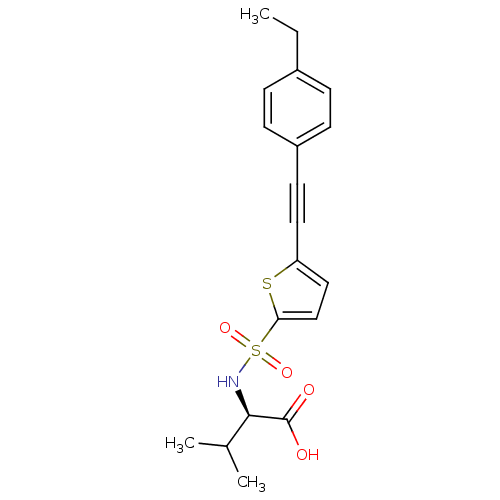
((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...)Show SMILES CCc1ccc(cc1)C#Cc1ccc(s1)S(=O)(=O)N[C@H](C(C)C)C(O)=O Show InChI InChI=1S/C19H21NO4S2/c1-4-14-5-7-15(8-6-14)9-10-16-11-12-17(25-16)26(23,24)20-18(13(2)3)19(21)22/h5-8,11-13,18,20H,4H2,1-3H3,(H,21,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) |
J Med Chem 42: 1723-38 (1999)
Article DOI: 10.1021/jm980514x
BindingDB Entry DOI: 10.7270/Q2KP81BQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50077155

((R)-2-[5-(4-Butyl-phenylethynyl)-thiophene-2-sulfo...)Show SMILES CCCCc1ccc(cc1)C#Cc1ccc(s1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H26N2O4S2/c1-2-3-6-19-9-11-20(12-10-19)13-14-22-15-16-26(34-22)35(32,33)29-25(27(30)31)17-21-18-28-24-8-5-4-7-23(21)24/h4-5,7-12,15-16,18,25,28-29H,2-3,6,17H2,1H3,(H,30,31)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) |
J Med Chem 42: 1723-38 (1999)
Article DOI: 10.1021/jm980514x
BindingDB Entry DOI: 10.7270/Q2KP81BQ |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50593027
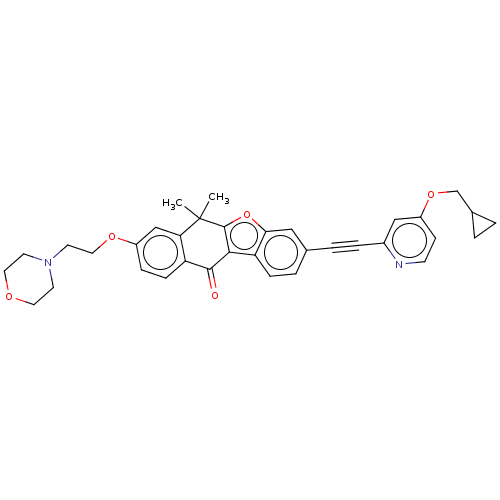
(CHEMBL5186340)Show SMILES CC1(C)c2oc3cc(ccc3c2C(=O)c2ccc(OCCN3CCOCC3)cc12)C#Cc1cc(OCC2CC2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01099
BindingDB Entry DOI: 10.7270/Q2FB56Z1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data