| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) |
|---|
| Ligand | BDBM484559 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Fluorescence Polarization Assay |
|---|
| Ki | 1.60±n/a nM |
|---|
| Citation |  Arasappan, A; Cox, JM; Debenham, JS; Guo, Z; He, J; Hussain, Z; Lai, Z; Li, D; Meng, D; Raghavan, S; Tyagarajan, S Substituted pyrazolo[3,4-d]pyrimidines as PDE9 inhibitors US Patent US10934294 Publication Date 3/2/2021 Arasappan, A; Cox, JM; Debenham, JS; Guo, Z; He, J; Hussain, Z; Lai, Z; Li, D; Meng, D; Raghavan, S; Tyagarajan, S Substituted pyrazolo[3,4-d]pyrimidines as PDE9 inhibitors US Patent US10934294 Publication Date 3/2/2021 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) |
|---|
| Name: | Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) |
|---|
| Synonyms: | High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) | PDE9A | PDE9A_HUMAN | Phosphodiesterase 9A (PDE9A2) | Phosphodiesterase 9A2 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 61702.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O76083-2 |
|---|
| Residue: | 533 |
|---|
| Sequence: | MGSGSSSYRPKAIYLDIDGRIQKVIFSKYCNSSDIMDLFCIATGLPRNTTISLLTTDDAM
VSIDPTMPANSERTPYKVRPVAIKQLSEREELIQSVLAQVAEQFSRAFKINELKAEVANH
LAVLEKRVELEGLKVVEIEKCKSDIKKMREELAARSSRTNCPCKYSFLDNHKKLTPRRDV
PTYPKYLLSPETIEALRKPTFDVWLWEPNEMLSCLEHMYHDLGLVRDFSINPVTLRRWLF
CVHDNYRNNPFHNFRHCFCVAQMMYSMVWLCSLQEKFSQTDILILMTAAICHDLDHPGYN
NTYQINARTELAVRYNDISPLENHHCAVAFQILAEPECNIFSNIPPDGFKQIRQGMITLI
LATDMARHAEIMDSFKEKMENFDYSNEEHMTLLKMILIKCCDISNEVRPMEVAEPWVDCL
LEEYFMQSDREKSEGLPVAPFMDRDKVTKATAQIGFIKFVLIPMFETVTKLFPMVEEIML
QPLWESRDRYEELKRIDDAMKELQKKTDSLTSGATEKSRERSRDVKNSEGDCA
|
|
|
|---|
| BDBM484559 |
|---|
| n/a |
|---|
| Name | BDBM484559 |
|---|
| Synonyms: | US10934294, Example 80 | US10934294, Example 81 | US11028092, Example 81 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H20FN9O |
|---|
| Mol. Mass. | 457.463 |
|---|
| SMILES | CC(c1cnc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@@H]1c1ncc(F)cn1 |r| |
|---|
| Structure | 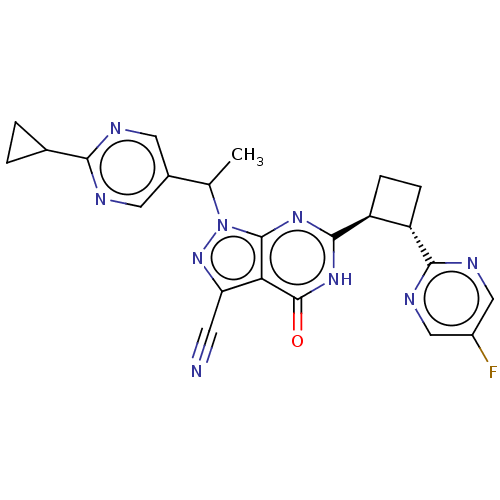
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Arasappan, A; Cox, JM; Debenham, JS; Guo, Z; He, J; Hussain, Z; Lai, Z; Li, D; Meng, D; Raghavan, S; Tyagarajan, S Substituted pyrazolo[3,4-d]pyrimidines as PDE9 inhibitors US Patent US10934294 Publication Date 3/2/2021
Arasappan, A; Cox, JM; Debenham, JS; Guo, Z; He, J; Hussain, Z; Lai, Z; Li, D; Meng, D; Raghavan, S; Tyagarajan, S Substituted pyrazolo[3,4-d]pyrimidines as PDE9 inhibitors US Patent US10934294 Publication Date 3/2/2021