 Found 5809 hits with Last Name = 'guo' and Initial = 'z'
Found 5809 hits with Last Name = 'guo' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484497
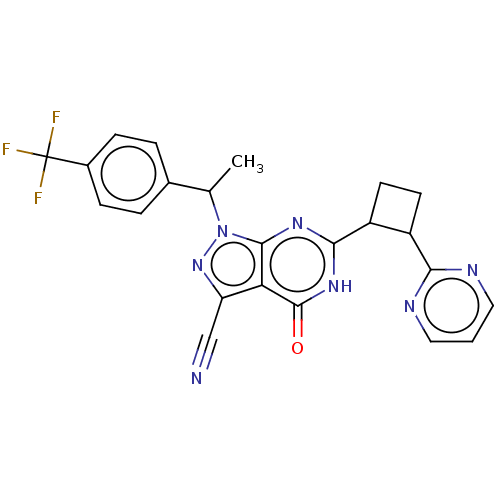
(US10934294, Example 19 | US10934294, Example 20 | ...)Show SMILES CC(c1ccc(cc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484570
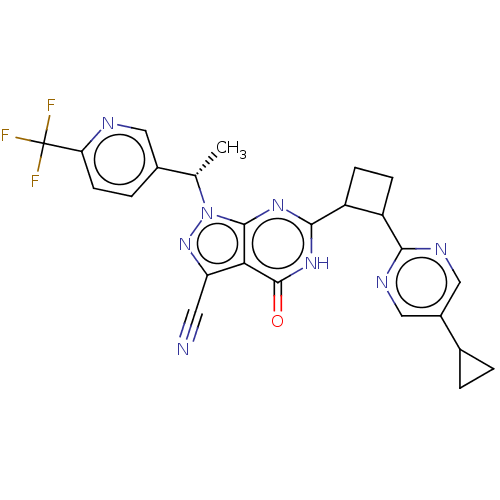
(US10934294, Example 91 | US10934294, Example 92 | ...)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(cn1)C1CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484541
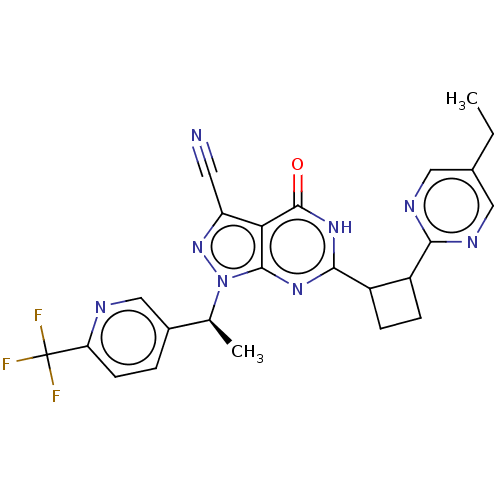
(US10934294, Example 62 | US11028092, Example 63)Show SMILES CCc1cnc(nc1)C1CCC1c1nc2n(nc(C#N)c2c(=O)[nH]1)[C@@H](C)c1ccc(nc1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484529

(US10934294, Example 50 | US10934294, Example 51 | ...)Show SMILES CC(c1ccc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484551

(US10934294, Example 72 | US10934294, Example 73 | ...)Show SMILES CC(c1ccc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(F)cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484572

(US10934294, Example 93)Show SMILES C[C@@H](c1cnc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@H]1c1ncccn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484593
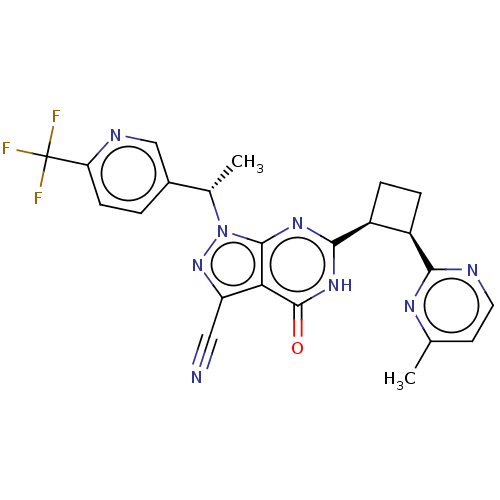
(US10934294, Example 111)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@H]1c1nccc(C)n1 Show InChI InChI=1S/C23H19F3N8O/c1-11-7-8-28-19(30-11)14-4-5-15(14)20-31-21-18(22(35)32-20)16(9-27)33-34(21)12(2)13-3-6-17(29-10-13)23(24,25)26/h3,6-8,10,12,14-15H,4-5H2,1-2H3,(H,31,32,35)/t12-,14+,15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484509
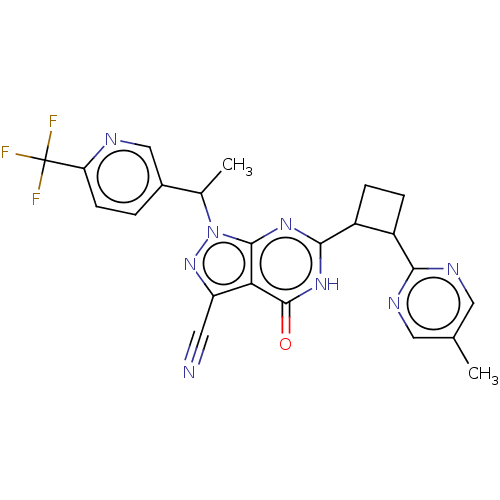
(US10934294, Example 31 | US10934294, Example 32 | ...)Show SMILES CC(c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(C)cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484596
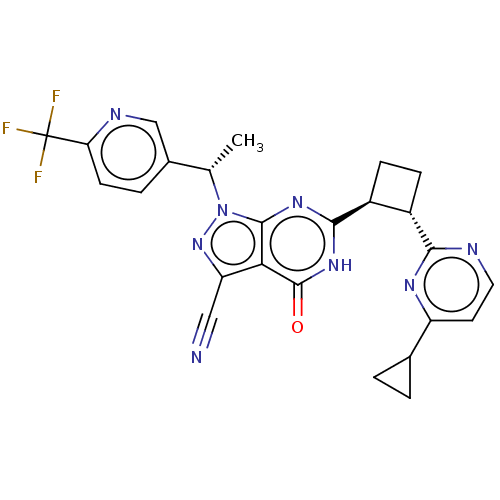
(US10934294, Example 114 | US11028092, Example 114)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@@H]1c1nccc(n1)C1CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484591

(US10934294, Example 110 | US11028092, Example 110)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@@H]1c1nccc(C)n1 Show InChI InChI=1S/C23H19F3N8O/c1-11-7-8-28-19(30-11)14-4-5-15(14)20-31-21-18(22(35)32-20)16(9-27)33-34(21)12(2)13-3-6-17(29-10-13)23(24,25)26/h3,6-8,10,12,14-15H,4-5H2,1-2H3,(H,31,32,35)/t12-,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26526
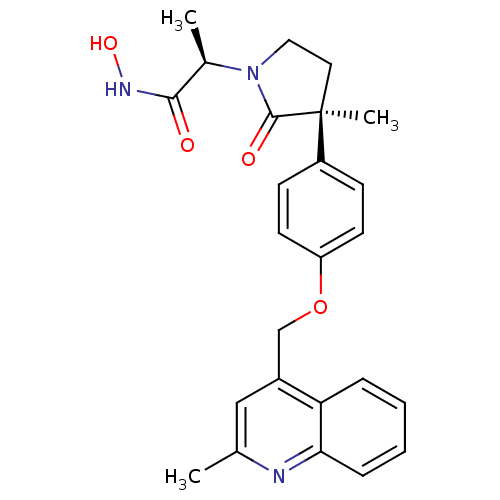
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332270
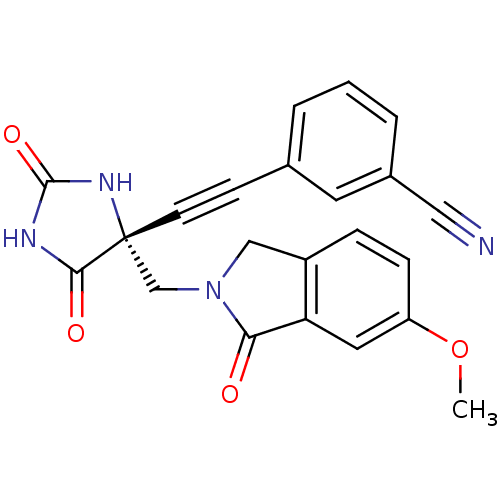
((R)-3-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(c3)C#N)C(=O)c2c1 |r| Show InChI InChI=1S/C22H16N4O4/c1-30-17-6-5-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)8-7-14-3-2-4-15(9-14)11-23/h2-6,9-10H,12-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332292
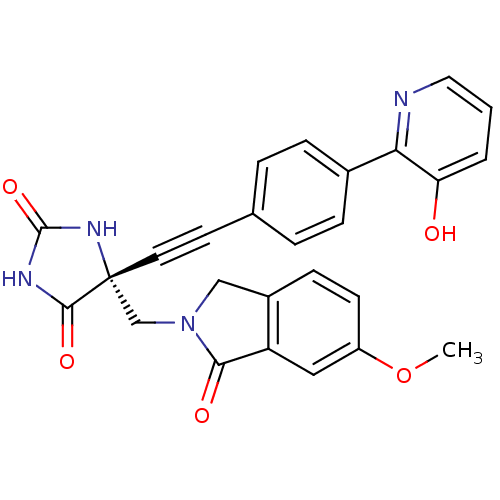
((R)-5-((4-(3-hydroxypyridin-2-yl)phenyl)ethynyl)-5...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3ncccc3O)C(=O)c2c1 |r| Show InChI InChI=1S/C26H20N4O5/c1-35-19-9-8-18-14-30(23(32)20(18)13-19)15-26(24(33)28-25(34)29-26)11-10-16-4-6-17(7-5-16)22-21(31)3-2-12-27-22/h2-9,12-13,31H,14-15H2,1H3,(H2,28,29,33,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484539
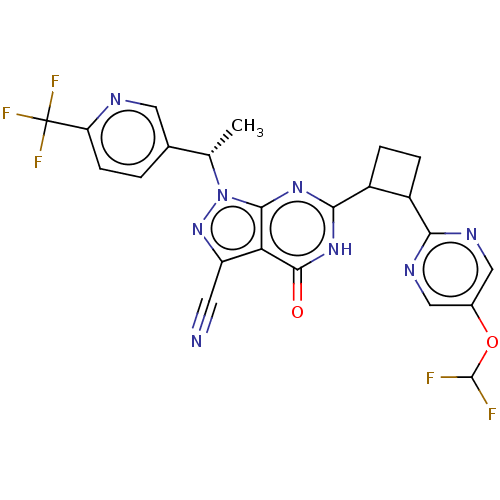
(US10934294, Example 60 | US10934294, Example 61 | ...)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(OC(F)F)cn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484559
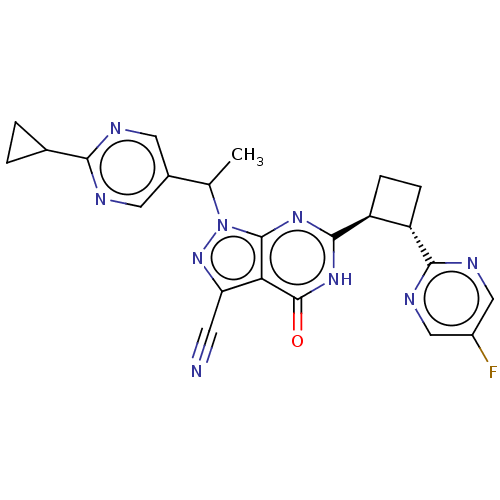
(US10934294, Example 80 | US10934294, Example 81 | ...)Show SMILES CC(c1cnc(nc1)C1CC1)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@@H]1c1ncc(F)cn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484484

(US10934294, Example 6 | US11028092, Example 6)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@@H]1c1ncccn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484489
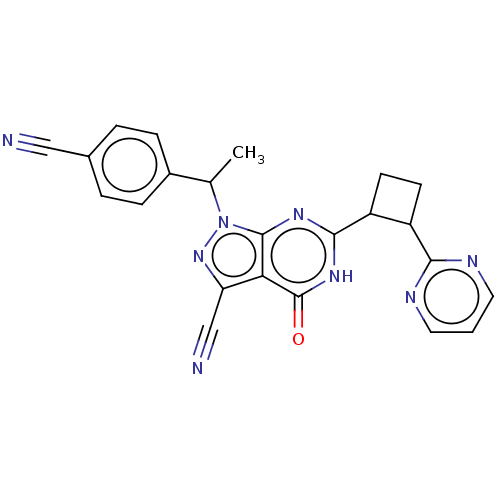
(US10934294, Example 11 | US10934294, Example 12 | ...)Show SMILES CC(c1ccc(cc1)C#N)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50325003
((R)-5-(4-(5-chloro-6-oxo-1,6-dihydropyridin-3-yl)p...)Show SMILES Fc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3ccc(cc3)-c3c[nH]c(=O)c(Cl)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H16ClFN4O4/c24-18-7-14(9-26-19(18)30)12-1-4-15(5-2-12)23(21(32)27-22(33)28-23)11-29-10-13-3-6-16(25)8-17(13)20(29)31/h1-9H,10-11H2,(H,26,30)(H2,27,28,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage |
Bioorg Med Chem Lett 20: 5286-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.134
BindingDB Entry DOI: 10.7270/Q26W9B8K |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM102669
(CHEMBL1288726 | US8541572, 976)Show SMILES CCN1CCN(CC1)c1ccc(O)c(n1)-c1ccc(cc1F)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H31FN6O5/c1-3-37-12-14-38(15-13-37)27-9-8-26(40)28(34-27)23-7-4-20(16-25(23)33)10-11-32(30(42)35-31(43)36-32)19-39-18-21-5-6-22(44-2)17-24(21)29(39)41/h4-9,16-17,40H,3,12-15,18-19H2,1-2H3,(H2,35,36,42,43)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484501

(US10934294, Example 23 | US10934294, Example 24 | ...)Show SMILES CC(c1cccc(c1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484537

(US10934294, Example 58 | US10934294, Example 59 | ...)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(Br)cn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484594

(BDBM502802 | US10934294, Example 112)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@@H]1c1nccc(n1)C(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332265

((R)-5-((2-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3F)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-15-7-6-14-11-25(18(26)16(14)10-15)12-21(19(27)23-20(28)24-21)9-8-13-4-2-3-5-17(13)22/h2-7,10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484517

(US10934294, Example 38 | US10934294, Example 39 | ...)Show SMILES CC(c1ccc(nc1)C(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncccn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332262
((R)-5-((6-methoxy-1-oxoisoindolin-2-yl)methyl)-5-(...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H17N3O4/c1-28-16-8-7-15-12-24(18(25)17(15)11-16)13-21(19(26)22-20(27)23-21)10-9-14-5-3-2-4-6-14/h2-8,11H,12-13H2,1H3,(H2,22,23,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26524

((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H24FN3O4/c29-22-12-17(14-28(27(30)34)15-21(28)26(33)32-35)10-11-25(22)36-16-19-13-24(18-6-2-1-3-7-18)31-23-9-5-4-8-20(19)23/h1-13,21,35H,14-16H2,(H2,30,34)(H,32,33)/t21-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26524

((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H24FN3O4/c29-22-12-17(14-28(27(30)34)15-21(28)26(33)32-35)10-11-25(22)36-16-19-13-24(18-6-2-1-3-7-18)31-23-9-5-4-8-20(19)23/h1-13,21,35H,14-16H2,(H2,30,34)(H,32,33)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292694
((1R,2S)-1-(3-fluoro-4-((2-(pyridin-3-yl)quinolin-4...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3cccnc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C27H23FN4O4/c28-21-10-16(12-27(26(29)34)13-20(27)25(33)32-35)7-8-24(21)36-15-18-11-23(17-4-3-9-30-14-17)31-22-6-2-1-5-19(18)22/h1-11,14,20,35H,12-13,15H2,(H2,29,34)(H,32,33)/t20-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibitory constant against binding of [125I]- IBZM to rat striatal membrane |
J Med Chem 31: 1039-43 (1988)
BindingDB Entry DOI: 10.7270/Q2C24X1V |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484542

(US10934294, Example 63 | US10934294, Example 97 | ...)Show SMILES COc1cnc(nc1)C1CCC1c1nc2n(nc(C#N)c2c(=O)[nH]1)[C@@H](C)c1ccc(nc1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292693
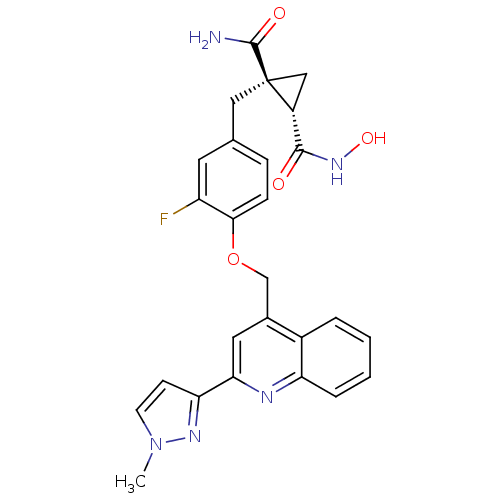
((1R,2S)-1-(3-fluoro-4-((2-(1-methyl-1H-pyrazol-3-y...)Show SMILES Cn1ccc(n1)-c1cc(COc2ccc(C[C@@]3(C[C@@H]3C(=O)NO)C(N)=O)cc2F)c2ccccc2n1 |r| Show InChI InChI=1S/C26H24FN5O4/c1-32-9-8-21(30-32)22-11-16(17-4-2-3-5-20(17)29-22)14-36-23-7-6-15(10-19(23)27)12-26(25(28)34)13-18(26)24(33)31-35/h2-11,18,35H,12-14H2,1H3,(H2,28,34)(H,31,33)/t18-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292691

((1R,2S)-1-(3-fluoro-4-((2-(pyrrolidin-1-yl)quinoli...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)N3CCCC3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C26H27FN4O4/c27-20-11-16(13-26(25(28)33)14-19(26)24(32)30-34)7-8-22(20)35-15-17-12-23(31-9-3-4-10-31)29-21-6-2-1-5-18(17)21/h1-2,5-8,11-12,19,34H,3-4,9-10,13-15H2,(H2,28,33)(H,30,32)/t19-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332289

((R)-5-((4-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C28H30N6O5/c1-3-32-12-14-33(15-13-32)24(31-38)20-6-4-19(5-7-20)10-11-28(26(36)29-27(37)30-28)18-34-17-21-8-9-22(39-2)16-23(21)25(34)35/h4-9,16,38H,3,12-15,17-18H2,1-2H3,(H2,29,30,36,37)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332288
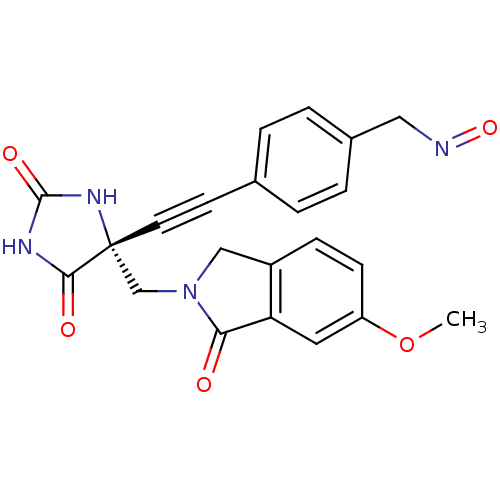
((R)-4-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(CN=O)cc3)C(=O)c2c1 |r| Show InChI InChI=1S/C22H18N4O5/c1-31-17-7-6-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)9-8-14-2-4-15(5-3-14)11-23-30/h2-7,10H,11-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332278
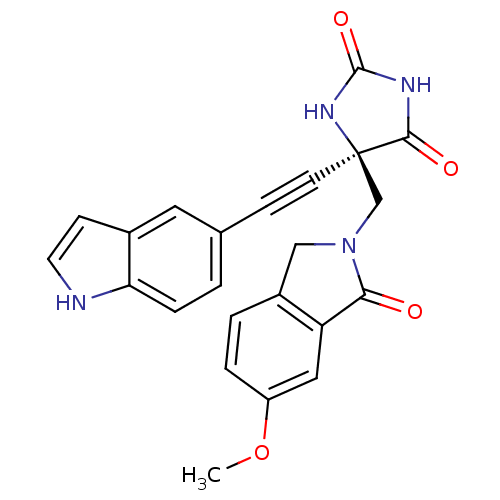
((R)-5-((1H-indol-5-yl)ethynyl)-5-((6-methoxy-1-oxo...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc4[nH]ccc4c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N4O4/c1-31-17-4-3-16-12-27(20(28)18(16)11-17)13-23(21(29)25-22(30)26-23)8-6-14-2-5-19-15(10-14)7-9-24-19/h2-5,7,9-11,24H,12-13H2,1H3,(H2,25,26,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484583
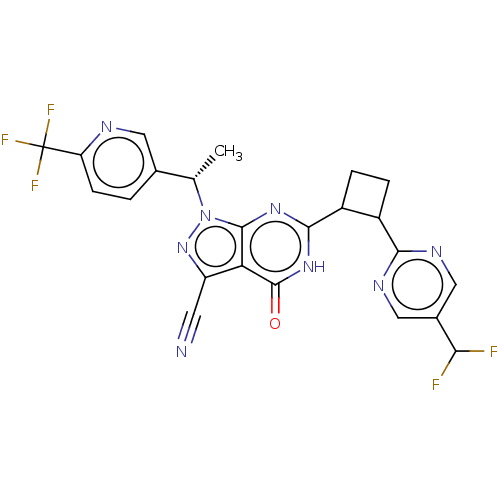
(US10934294, Example 102 | US10934294, Example 103 ...)Show SMILES C[C@@H](c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(cn1)C(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26525
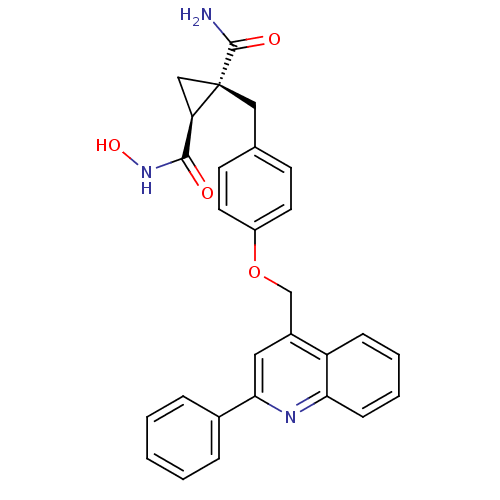
((1S,2R)-1-N-hydroxy-2-({4-[(2-phenylquinolin-4-yl)...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H25N3O4/c29-27(33)28(16-23(28)26(32)31-34)15-18-10-12-21(13-11-18)35-17-20-14-25(19-6-2-1-3-7-19)30-24-9-5-4-8-22(20)24/h1-14,23,34H,15-17H2,(H2,29,33)(H,31,32)/t23-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26525

((1S,2R)-1-N-hydroxy-2-({4-[(2-phenylquinolin-4-yl)...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H25N3O4/c29-27(33)28(16-23(28)26(32)31-34)15-18-10-12-21(13-11-18)35-17-20-14-25(19-6-2-1-3-7-19)30-24-9-5-4-8-22(20)24/h1-14,23,34H,15-17H2,(H2,29,33)(H,31,32)/t23-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332290
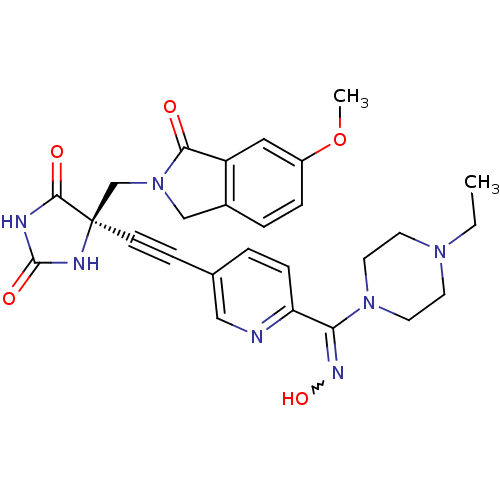
((R)-5-((6-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cn1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C27H29N7O5/c1-3-32-10-12-33(13-11-32)23(31-38)22-7-4-18(15-28-22)8-9-27(25(36)29-26(37)30-27)17-34-16-19-5-6-20(39-2)14-21(19)24(34)35/h4-7,14-15,38H,3,10-13,16-17H2,1-2H3,(H2,29,30,36,37)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332279
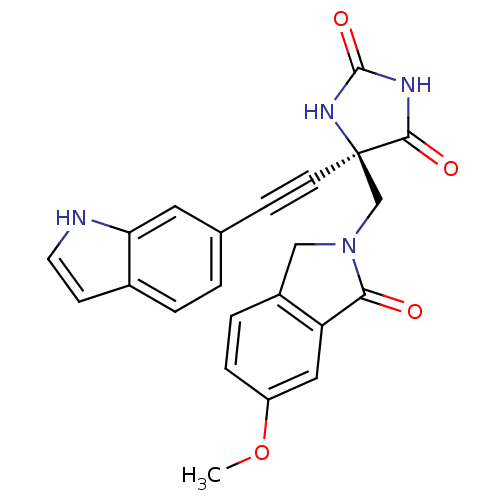
((R)-5-((1H-indol-6-yl)ethynyl)-5-((6-methoxy-1-oxo...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc4cc[nH]c4c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N4O4/c1-31-17-5-4-16-12-27(20(28)18(16)11-17)13-23(21(29)25-22(30)26-23)8-6-14-2-3-15-7-9-24-19(15)10-14/h2-5,7,9-11,24H,12-13H2,1H3,(H2,25,26,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484480

(US10934294, Example 2 | US11028092, Example 2)Show SMILES C[C@@H](c1ccc(F)cc1)n1nc(C#N)c2c1nc([nH]c2=O)[C@H]1CC[C@@H]1c1ncccn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)
(Homo sapiens (Human)) | BDBM484587
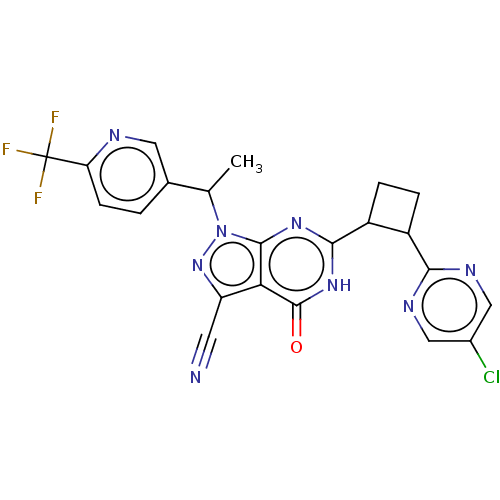
(US10934294, Example 106 | US10934294, Example 107 ...)Show SMILES CC(c1ccc(nc1)C(F)(F)F)n1nc(C#N)c2c1nc([nH]c2=O)C1CCC1c1ncc(Cl)cn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... |
US Patent US10934294 (2021)
BindingDB Entry DOI: 10.7270/Q2C250JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332264
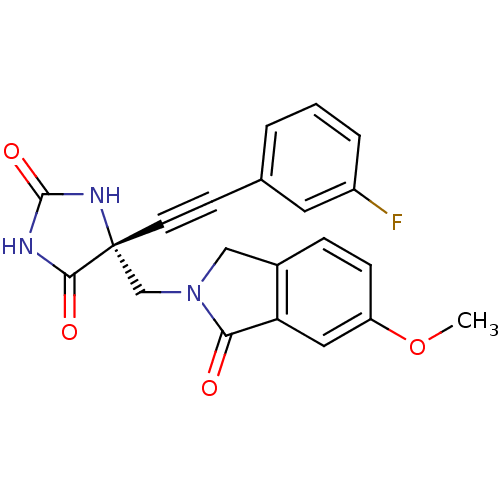
((R)-5-((3-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(F)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-16-6-5-14-11-25(18(26)17(14)10-16)12-21(19(27)23-20(28)24-21)8-7-13-3-2-4-15(22)9-13/h2-6,9-10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343977
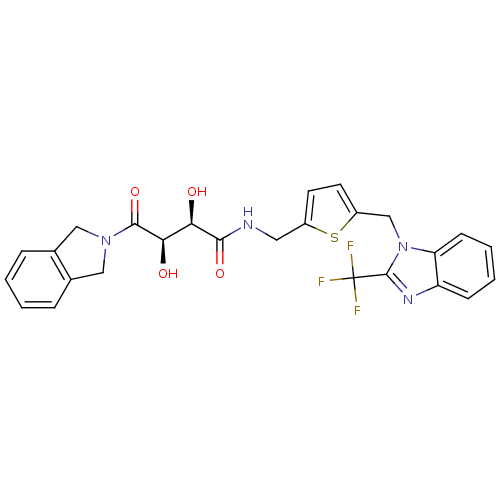
((2R,3R)-2,3-dihydroxy-4-(isoindolin-2-yl)-4-oxo-N-...)Show SMILES O[C@H]([C@@H](O)C(=O)N1Cc2ccccc2C1)C(=O)NCc1ccc(Cn2c(nc3ccccc23)C(F)(F)F)s1 |r| Show InChI InChI=1S/C26H23F3N4O4S/c27-26(28,29)25-31-19-7-3-4-8-20(19)33(25)14-18-10-9-17(38-18)11-30-23(36)21(34)22(35)24(37)32-12-15-5-1-2-6-16(15)13-32/h1-10,21-22,34-35H,11-14H2,(H,30,36)/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50325002
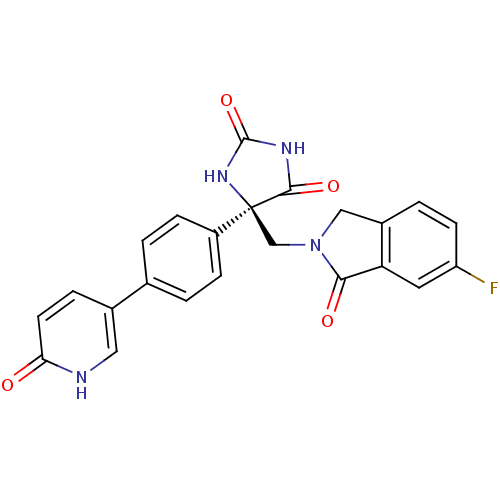
((R)-5-((6-fluoro-1-oxoisoindolin-2-yl)methyl)-5-(4...)Show SMILES Fc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3ccc(cc3)-c3ccc(=O)[nH]c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H17FN4O4/c24-17-7-3-15-11-28(20(30)18(15)9-17)12-23(21(31)26-22(32)27-23)16-5-1-13(2-6-16)14-4-8-19(29)25-10-14/h1-10H,11-12H2,(H,25,29)(H2,26,27,31,32)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage |
Bioorg Med Chem Lett 20: 5286-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.134
BindingDB Entry DOI: 10.7270/Q26W9B8K |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50243056
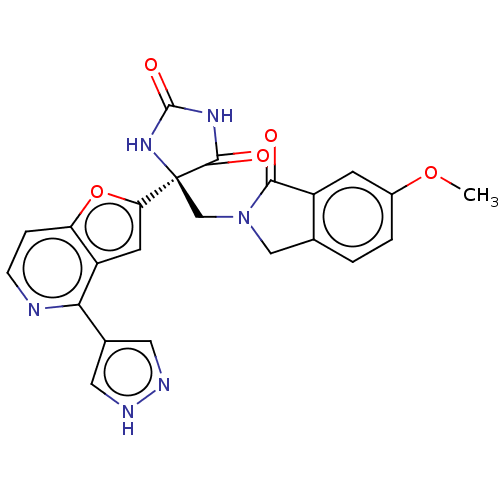
(CHEMBL4085232)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3cc4c(nccc4o3)-c3cn[nH]c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N6O5/c1-33-14-3-2-12-10-29(20(30)15(12)6-14)11-23(21(31)27-22(32)28-23)18-7-16-17(34-18)4-5-24-19(16)13-8-25-26-9-13/h2-9H,10-11H2,1H3,(H,25,26)(H2,27,28,31,32)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain TACE (unknown origin) by FRET assay |
Bioorg Med Chem Lett 27: 3037-3042 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.062
BindingDB Entry DOI: 10.7270/Q2319Z8G |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50067485
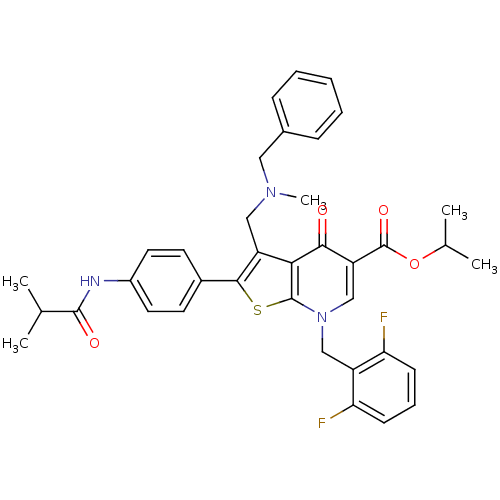
(3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...)Show SMILES CC(C)OC(=O)c1cn(Cc2c(F)cccc2F)c2sc(c(CN(C)Cc3ccccc3)c2c1=O)-c1ccc(NC(=O)C(C)C)cc1 Show InChI InChI=1S/C37H37F2N3O4S/c1-22(2)35(44)40-26-16-14-25(15-17-26)34-28(19-41(5)18-24-10-7-6-8-11-24)32-33(43)29(37(45)46-23(3)4)21-42(36(32)47-34)20-27-30(38)12-9-13-31(27)39/h6-17,21-23H,18-20H2,1-5H3,(H,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 13: 3617-22 (2003)
BindingDB Entry DOI: 10.7270/Q24X576N |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122654

(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CONC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C36H31F2N5O4S/c1-41(20-23-10-5-3-6-11-23)21-28-31-33(44)43(26-12-7-4-8-13-26)36(46)42(22-27-29(37)14-9-15-30(27)38)34(31)48-32(28)24-16-18-25(19-17-24)39-35(45)40-47-2/h3-19H,20-22H2,1-2H3,(H2,39,40,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human gonadotropin-releasing hormone receptor |
Bioorg Med Chem Lett 13: 3617-22 (2003)
BindingDB Entry DOI: 10.7270/Q24X576N |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213665
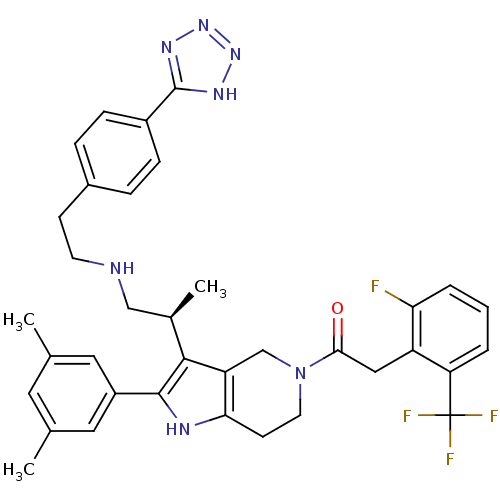
((S)-1-(3-(1-(4-(1H-tetrazol-5-yl)phenethylamino)pr...)Show SMILES C[C@H](CNCCc1ccc(cc1)-c1nnn[nH]1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F |r| Show InChI InChI=1S/C36H37F4N7O/c1-21-15-22(2)17-26(16-21)34-33(23(3)19-41-13-11-24-7-9-25(10-8-24)35-43-45-46-44-35)28-20-47(14-12-31(28)42-34)32(48)18-27-29(36(38,39)40)5-4-6-30(27)37/h4-10,15-17,23,41-42H,11-14,18-20H2,1-3H3,(H,43,44,45,46)/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50243045
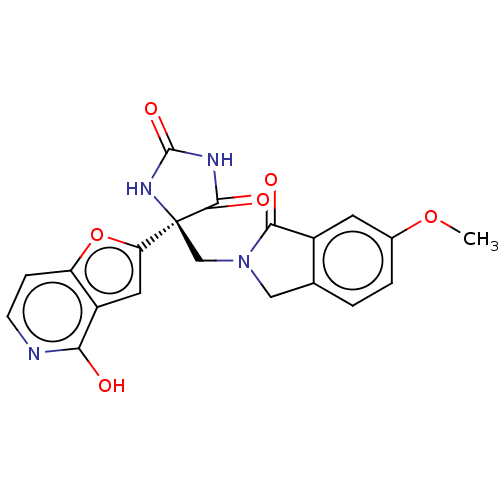
(CHEMBL4095412)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3cc4c(O)nccc4o3)C(=O)c2c1 |r| Show InChI InChI=1S/C20H16N4O6/c1-29-11-3-2-10-8-24(17(26)12(10)6-11)9-20(18(27)22-19(28)23-20)15-7-13-14(30-15)4-5-21-16(13)25/h2-7H,8-9H2,1H3,(H,21,25)(H2,22,23,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain TACE (unknown origin) by FRET assay |
Bioorg Med Chem Lett 27: 3037-3042 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.062
BindingDB Entry DOI: 10.7270/Q2319Z8G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data