| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone H3.1 |
|---|
| Ligand | BDBM50248375 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Histone H3 Acetylation Assay |
|---|
| EC50 | 1700±n/a nM |
|---|
| Citation |  Chen, D; Song, HY; Sun, ET; Yu, N; Zou, Y Benzimidazole derivatives: preparation and pharmaceutical applications US Patent US10201527 Publication Date 2/12/2019 Chen, D; Song, HY; Sun, ET; Yu, N; Zou, Y Benzimidazole derivatives: preparation and pharmaceutical applications US Patent US10201527 Publication Date 2/12/2019 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone H3.1 |
|---|
| Name: | Histone H3.1 |
|---|
| Synonyms: | H31_HUMAN | H3C1 | H3FA | H3FB | H3FC HIST1H3C | H3FD | H3FF | H3FH | H3FI | H3FJ | H3FK | H3FL | HIST1H3A | HIST1H3B | HIST1H3D | HIST1H3E | HIST1H3F | HIST1H3G | HIST1H3H | HIST1H3I | HIST1H3J |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 15425.07 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 136 |
|---|
| Sequence: | MARTKQTARKSTGGKAPRKQLATKAARKSAPATGGVKKPHRYRPGTVALREIRRYQKSTE
LLIRKLPFQRLVREIAQDFKTDLRFQSSAVMALQEACEAYLVGLFEDTNLCAIHAKRVTI
MPKDIQLARRIRGERA
|
|
|
|---|
| BDBM50248375 |
|---|
| n/a |
|---|
| Name | BDBM50248375 |
|---|
| Synonyms: | CHEMBL491688 | N-hydroxy-3-(2-phenethyl-1-(3,4,5-trimethoxybenzyl)-1H-benzo[d]imidazol-5-yl)acrylamide | US10201527, Compound 2 | US10736881, Compound 2 | US8551988, 2 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H29N3O5 |
|---|
| Mol. Mass. | 487.547 |
|---|
| SMILES | COc1cc(Cn2c(CCc3ccccc3)nc3cc(\C=C\C(=O)NO)ccc23)cc(OC)c1OC |
|---|
| Structure | 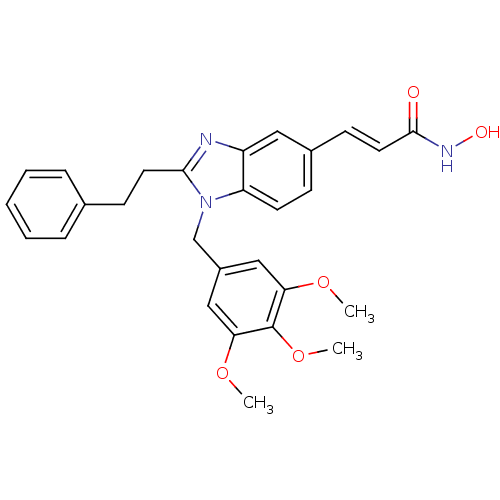
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chen, D; Song, HY; Sun, ET; Yu, N; Zou, Y Benzimidazole derivatives: preparation and pharmaceutical applications US Patent US10201527 Publication Date 2/12/2019
Chen, D; Song, HY; Sun, ET; Yu, N; Zou, Y Benzimidazole derivatives: preparation and pharmaceutical applications US Patent US10201527 Publication Date 2/12/2019