| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50466485 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1793318 (CHEMBL4265237) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Guo, B; Guo, S; Huang, J; Li, J; Li, J; Chen, Q; Zhou, X; Xie, X; Yang, Y Design and optimization of 2,3-dihydrobenzo[b][1,4]dioxine propanoic acids as novel GPR40 agonists with improved pharmacokinetic and safety profiles. Bioorg Med Chem26:5780-5791 (2018) [PubMed] Article Guo, B; Guo, S; Huang, J; Li, J; Li, J; Chen, Q; Zhou, X; Xie, X; Yang, Y Design and optimization of 2,3-dihydrobenzo[b][1,4]dioxine propanoic acids as novel GPR40 agonists with improved pharmacokinetic and safety profiles. Bioorg Med Chem26:5780-5791 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50466485 |
|---|
| n/a |
|---|
| Name | BDBM50466485 |
|---|
| Synonyms: | CHEMBL4288001 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H19ClO5 |
|---|
| Mol. Mass. | 386.826 |
|---|
| SMILES | CC#C[C@@H](CC(O)=O)c1ccc(OC[C@H]2COc3ccc(Cl)cc3O2)cc1 |r| |
|---|
| Structure | 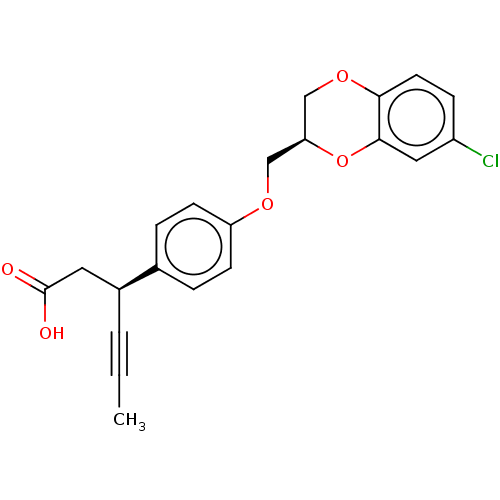
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Guo, B; Guo, S; Huang, J; Li, J; Li, J; Chen, Q; Zhou, X; Xie, X; Yang, Y Design and optimization of 2,3-dihydrobenzo[b][1,4]dioxine propanoic acids as novel GPR40 agonists with improved pharmacokinetic and safety profiles. Bioorg Med Chem26:5780-5791 (2018) [PubMed] Article
Guo, B; Guo, S; Huang, J; Li, J; Li, J; Chen, Q; Zhou, X; Xie, X; Yang, Y Design and optimization of 2,3-dihydrobenzo[b][1,4]dioxine propanoic acids as novel GPR40 agonists with improved pharmacokinetic and safety profiles. Bioorg Med Chem26:5780-5791 (2018) [PubMed] Article