

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040861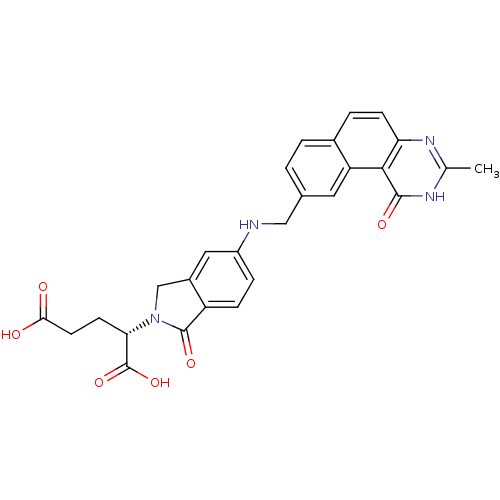 ((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of TS by spectrophotometry | Bioorg Med Chem 19: 3585-94 (2011) Article DOI: 10.1016/j.bmc.2011.03.067 BindingDB Entry DOI: 10.7270/Q22V2GGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004178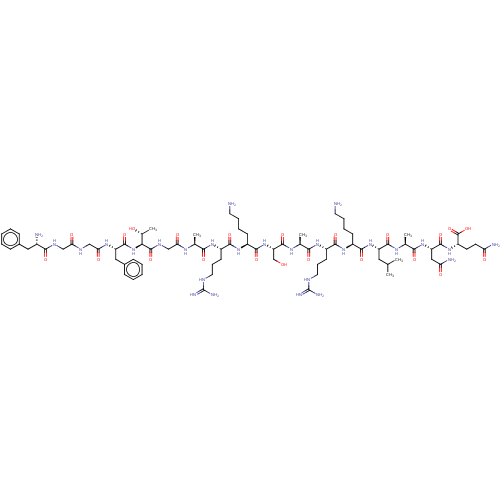 (Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP Curated by ChEMBL | Assay Description Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 | Bioorg Med Chem Lett 14: 5045-50 (2004) Article DOI: 10.1016/j.bmcl.2004.08.001 BindingDB Entry DOI: 10.7270/Q2TM7BV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220380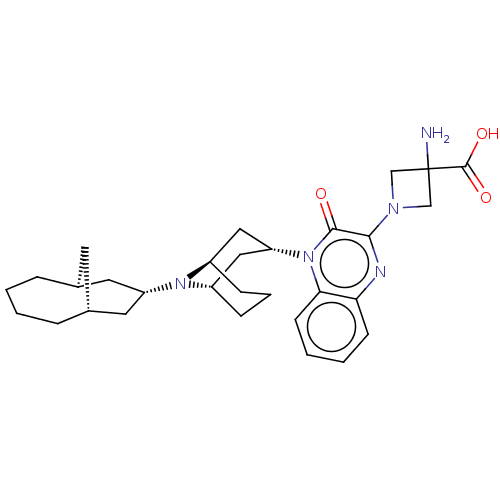 (US9290488, ZA01) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50594944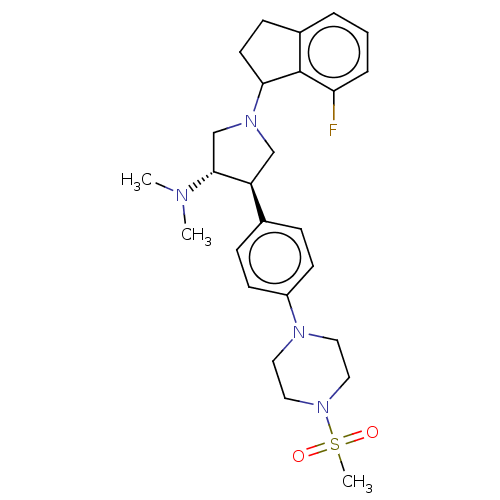 (CHEMBL5181703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114144 BindingDB Entry DOI: 10.7270/Q2DN4921 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220382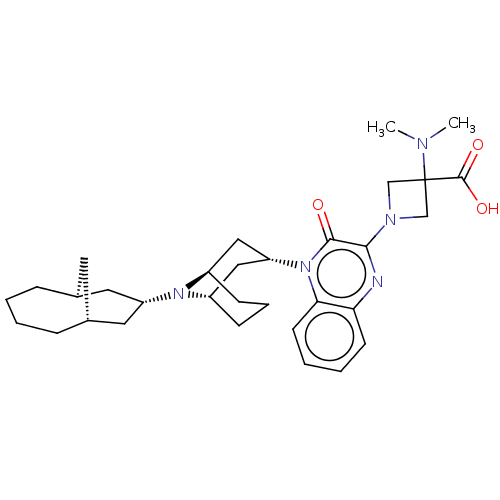 (US9290488, ZA03) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50512458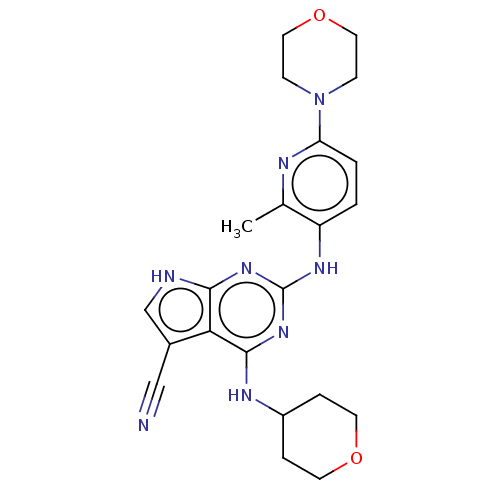 (CHEMBL4450240) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of MPS1 (510 to 857 residues) catalytic domain (unknown origin) expressed in Escherichia coli after 15 mins by mass-spectrometry analysis | Eur J Med Chem 175: 247-268 (2019) Article DOI: 10.1016/j.ejmech.2019.04.047 BindingDB Entry DOI: 10.7270/Q21V5J8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50512455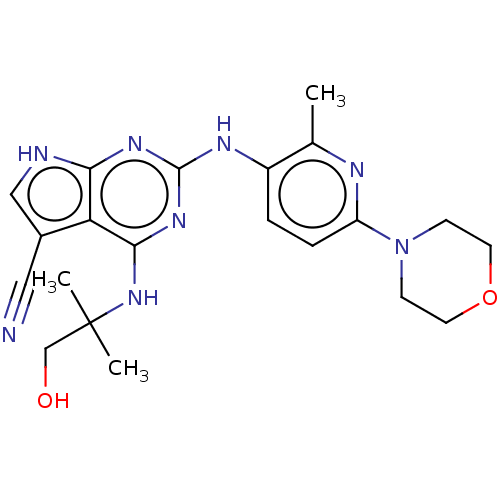 (CHEMBL4436510) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of MPS1 (510 to 857 residues) catalytic domain (unknown origin) expressed in Escherichia coli after 15 mins by mass-spectrometry analysis | Eur J Med Chem 175: 247-268 (2019) Article DOI: 10.1016/j.ejmech.2019.04.047 BindingDB Entry DOI: 10.7270/Q21V5J8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280750 (CHEMBL4160361) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAS guanyl-releasing protein 1 (Homo sapiens (Human)) | BDBM50244449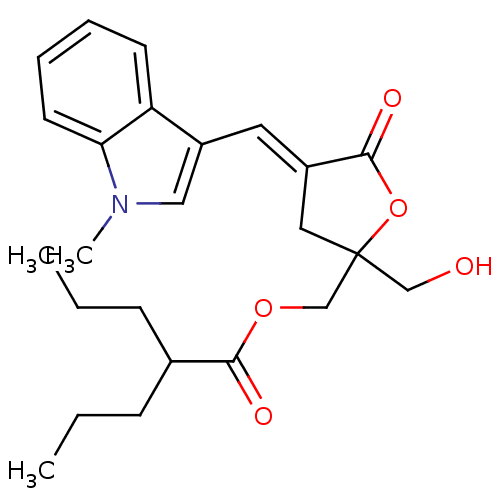 ((E)-(2-(hydroxymethyl)-4-((1-methyl-1H-indol-3-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Industrial Technology Curated by ChEMBL | Assay Description Displacement of [20-3H]phorbol 12,13-dibutyrate from RasGRP1 (unknown origin) in the presence of porcine brain phosphatidylserine by scintillation co... | Bioorg Med Chem 25: 2971-2980 (2017) Article DOI: 10.1016/j.bmc.2017.03.022 BindingDB Entry DOI: 10.7270/Q28P62P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172430 (US9090618, ZA53 | US9598411, Ref. No. ZA53) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM172430 (US9090618, ZA53 | US9598411, Ref. No. ZA53) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50244449 ((E)-(2-(hydroxymethyl)-4-((1-methyl-1H-indol-3-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220363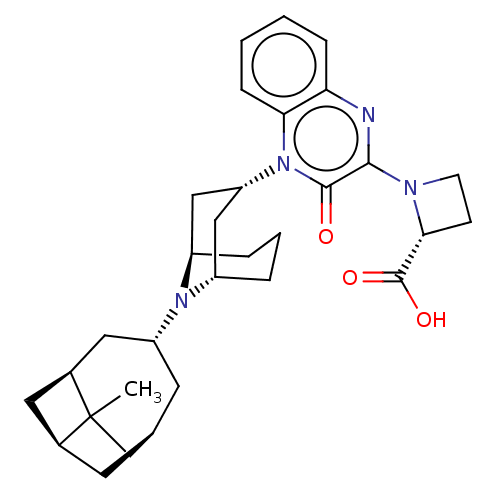 (US9290488, B20a(i)) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.890 | -51.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172419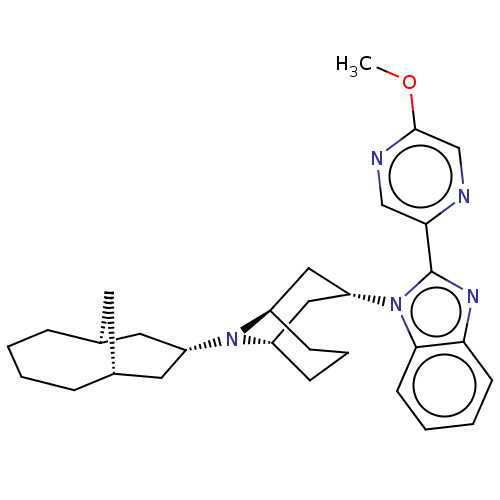 (US9090618, ZA42 | US9598411, Ref. No. ZA42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM172419 (US9090618, ZA42 | US9598411, Ref. No. ZA42) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.920 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM303064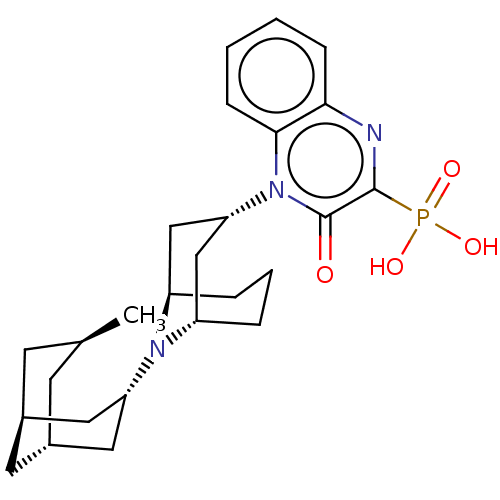 (US9598447, Ref No. FF) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in ... | US Patent US9598447 (2017) BindingDB Entry DOI: 10.7270/Q2P27168 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280765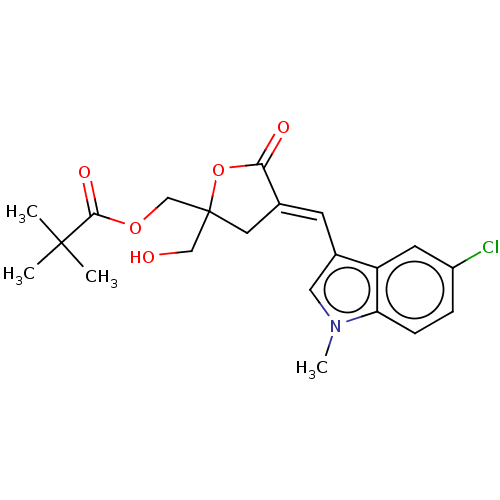 (CHEMBL4160647) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220366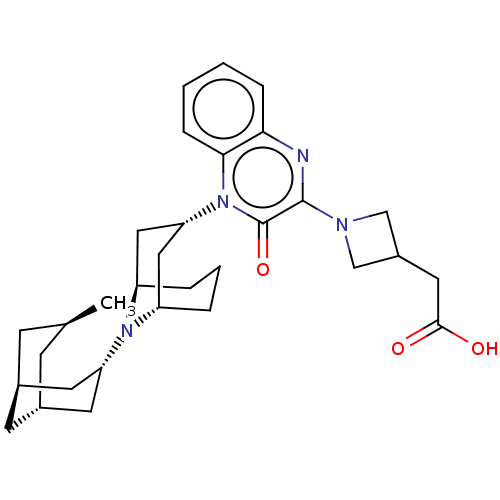 (US9290488, B34a) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.03 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280759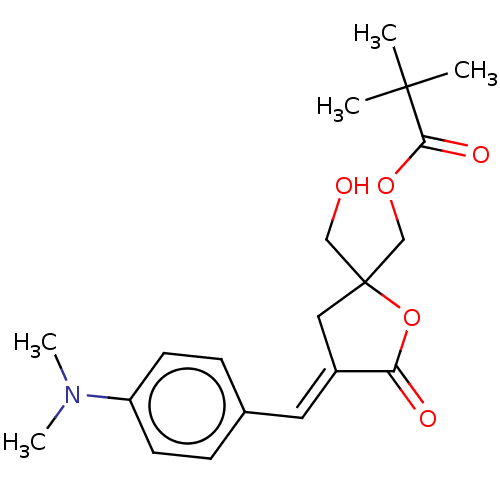 (CHEMBL4162516) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280768 (CHEMBL4163130) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM172418 (US9090618, ZA41 | US9598411, Ref. No. ZA41) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.15 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172418 (US9090618, ZA41 | US9598411, Ref. No. ZA41) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50184825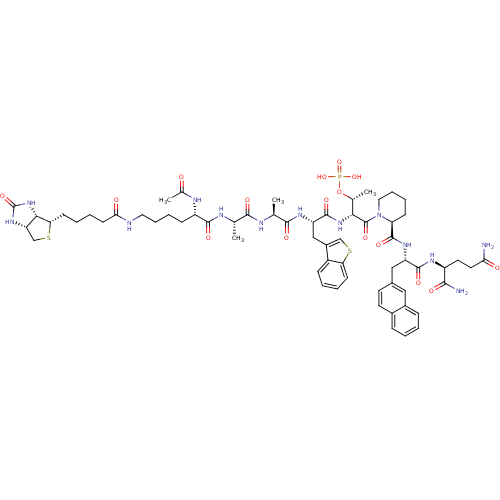 (Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bth-D-Thr(PO3H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of human Pin1 PPIase Activity by protease free PPIase assay | J Med Chem 49: 2147-50 (2006) Article DOI: 10.1021/jm060036n BindingDB Entry DOI: 10.7270/Q2S46RJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced 45Ca2+ uptake incubated for 5 mins by liquid sc... | Bioorg Med Chem Lett 26: 3603-7 (2016) Article DOI: 10.1016/j.bmcl.2016.06.010 BindingDB Entry DOI: 10.7270/Q2QC05FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220379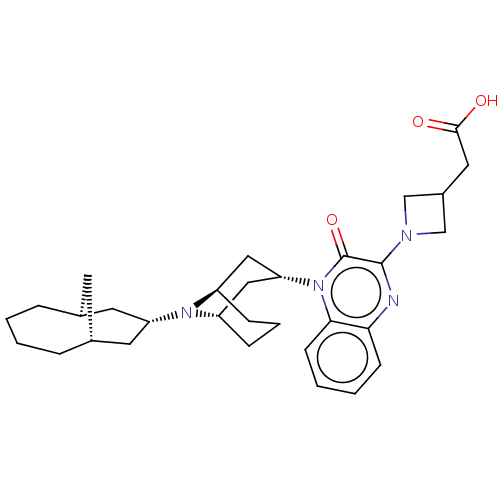 (US9290488, L29b) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.32 | -50.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220375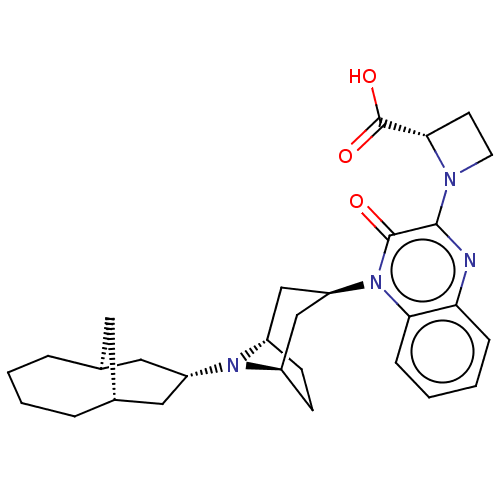 (US9290488, K2b(ii)) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.46 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280751 (CHEMBL4174397) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50607921 (CHEMBL5278357) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50607921 (CHEMBL5278357) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50607921 (CHEMBL5278357) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50607921 (CHEMBL5278357) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280773 (CHEMBL4174723) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280749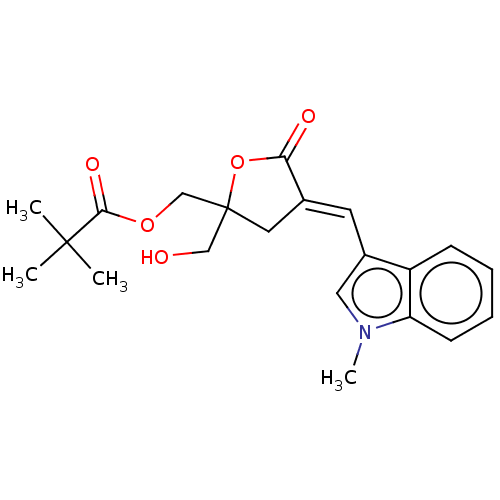 (CHEMBL4169454) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280756 (CHEMBL4167589) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50513547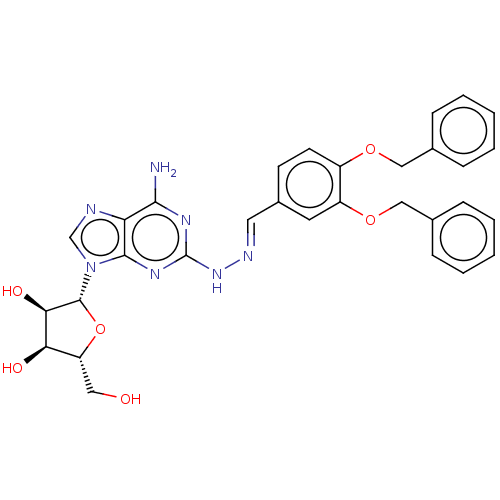 (CHEMBL4471366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human cloned adenosine receptor A2A expressed in HEK-293 cell membrane incubated for 60 mins by microbeta counting ... | Eur J Med Chem 179: 310-324 (2019) Article DOI: 10.1016/j.ejmech.2019.06.050 BindingDB Entry DOI: 10.7270/Q24B34NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM172382 (US9090618, H27b(i) | US9598411, Ref. No. H27b(i)) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.90 | -49.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172382 (US9090618, H27b(i) | US9598411, Ref. No. H27b(i)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280771 (CHEMBL4160540) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220384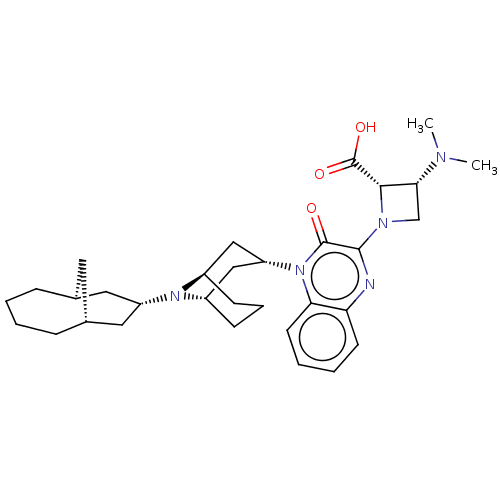 (US9290488, ZA05) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.97 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220385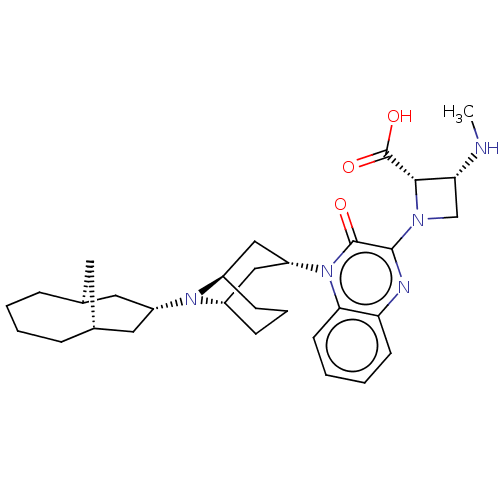 (US9290488, ZA06) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.98 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220383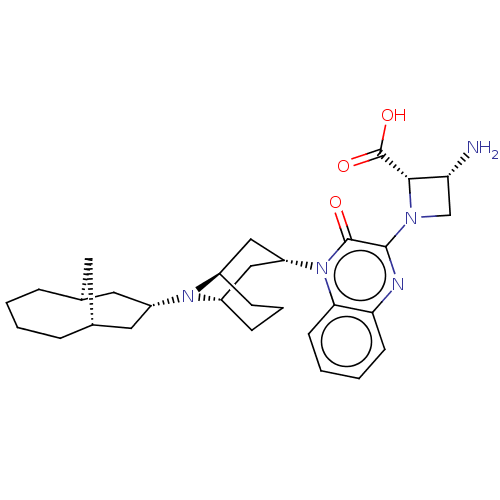 (US9290488, ZA04) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.98 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50513531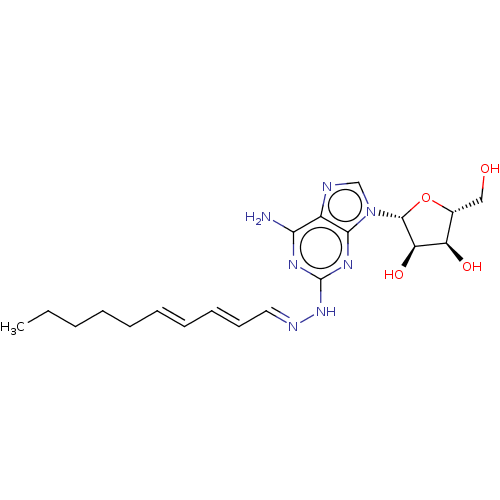 (CHEMBL4435740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human cloned adenosine receptor A1 expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta counting meth... | Eur J Med Chem 179: 310-324 (2019) Article DOI: 10.1016/j.ejmech.2019.06.050 BindingDB Entry DOI: 10.7270/Q24B34NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM172417 (US9090618, ZA40 | US9598411, Ref. No. ZA40) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM172433 (US9090618, ZA56 | US9598411, Ref. No. ZA56) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.05 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 ug membrane protein in a fin... | US Patent US9090618 (2015) BindingDB Entry DOI: 10.7270/Q2G73CGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172433 (US9090618, ZA56 | US9598411, Ref. No. ZA56) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM147191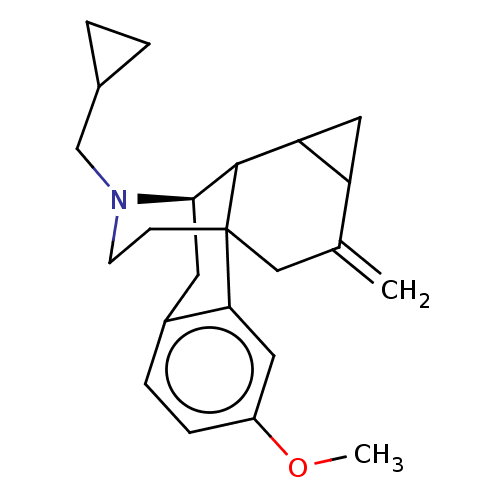 (US8957084, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.19 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant... | US Patent US8957084 (2015) BindingDB Entry DOI: 10.7270/Q21Z433P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553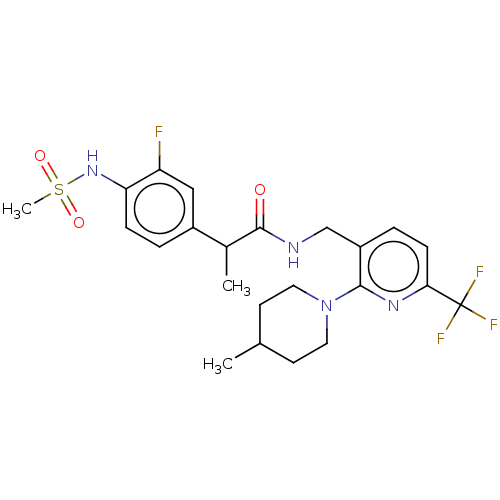 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced 45Ca2+ uptake incubated for 5 mins by liquid sc... | Bioorg Med Chem Lett 26: 3603-7 (2016) Article DOI: 10.1016/j.bmcl.2016.06.010 BindingDB Entry DOI: 10.7270/Q2QC05FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM172417 (US9090618, ZA40 | US9598411, Ref. No. ZA40) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P. US Patent | Assay Description δ-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement assays used 0.2 nM [3H]-Naltrindole (NEN; 33.0 Ci/mmole) with 10-20 &#... | US Patent US9598411 (2017) BindingDB Entry DOI: 10.7270/Q2ZG6V9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ras guanyl-releasing protein 3 (Homo sapiens (Human)) | BDBM50280752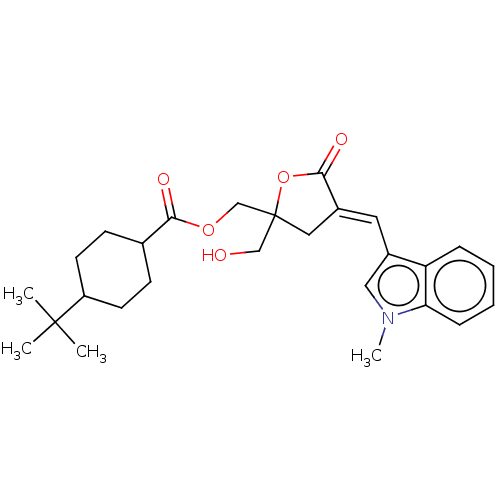 (CHEMBL4161221) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from full length MBP-tagged human RasGRP3 expressed in Escherichia coli BL21 (DE3) after 5 mins by scintillation counting | J Med Chem 61: 6261-6276 (2018) Article DOI: 10.1021/acs.jmedchem.8b00661 BindingDB Entry DOI: 10.7270/Q28P632D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM220362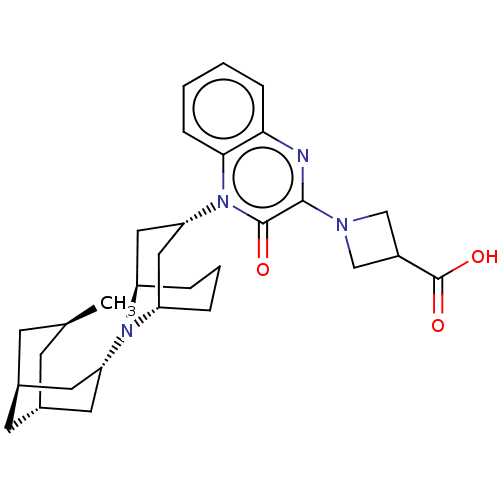 (US9290488, B12a) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.21 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma L.P. US Patent | Assay Description Radioligand binding assays (screening and dose-displacement) used 0.1 nM [3H]-nociceptin (NEN; 87.7 Ci/mmole) with 10-20 μg membrane protein in a ... | US Patent US9290488 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5695 total ) | Next | Last >> |