| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50500760 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1549817 (CHEMBL3757701) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Makhaeva, GF; Boltneva, NP; Lushchekina, SV; Serebryakova, OG; Stupina, TS; Terentiev, AA; Serkov, IV; Proshin, AN; Bachurin, SO; Richardson, RJ Synthesis, molecular docking and biological evaluation of N,N-disubstituted 2-aminothiazolines as a new class of butyrylcholinesterase and carboxylesterase inhibitors. Bioorg Med Chem24:1050-62 (2016) [PubMed] Article Makhaeva, GF; Boltneva, NP; Lushchekina, SV; Serebryakova, OG; Stupina, TS; Terentiev, AA; Serkov, IV; Proshin, AN; Bachurin, SO; Richardson, RJ Synthesis, molecular docking and biological evaluation of N,N-disubstituted 2-aminothiazolines as a new class of butyrylcholinesterase and carboxylesterase inhibitors. Bioorg Med Chem24:1050-62 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67792.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22303 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPV
SAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSM
NYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASV
GMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTEL
VACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVG
VVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPE
DPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGY
EIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQ
YVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQDRCSDL
|
|
|
|---|
| BDBM50500760 |
|---|
| n/a |
|---|
| Name | BDBM50500760 |
|---|
| Synonyms: | CHEMBL3754622 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H18ClI2N3S |
|---|
| Mol. Mass. | 585.672 |
|---|
| SMILES | I.Clc1ccc(CN(Cc2cccnc2)C2=NCC(CI)S2)cc1 |t:15| |
|---|
| Structure | 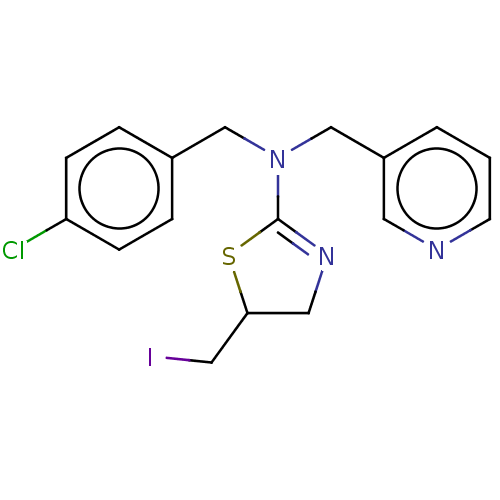
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Makhaeva, GF; Boltneva, NP; Lushchekina, SV; Serebryakova, OG; Stupina, TS; Terentiev, AA; Serkov, IV; Proshin, AN; Bachurin, SO; Richardson, RJ Synthesis, molecular docking and biological evaluation of N,N-disubstituted 2-aminothiazolines as a new class of butyrylcholinesterase and carboxylesterase inhibitors. Bioorg Med Chem24:1050-62 (2016) [PubMed] Article
Makhaeva, GF; Boltneva, NP; Lushchekina, SV; Serebryakova, OG; Stupina, TS; Terentiev, AA; Serkov, IV; Proshin, AN; Bachurin, SO; Richardson, RJ Synthesis, molecular docking and biological evaluation of N,N-disubstituted 2-aminothiazolines as a new class of butyrylcholinesterase and carboxylesterase inhibitors. Bioorg Med Chem24:1050-62 (2016) [PubMed] Article