| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM50169304 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_302654 (CHEMBL839943) |
|---|
| Ki | 3.3±n/a nM |
|---|
| Citation |  Matasi, JJ; Caldwell, JP; Zhang, H; Fawzi, A; Cohen-Williams, ME; Varty, GB; Tulshian, DB 2-(2-Furanyl)-7-phenyl[1,2,4]triazolo[1,5-c]pyrimidin-5-amine analogs: highly potent, orally active, adenosine A2A antagonists. Part 1. Bioorg Med Chem Lett15:3670-4 (2005) [PubMed] Article Matasi, JJ; Caldwell, JP; Zhang, H; Fawzi, A; Cohen-Williams, ME; Varty, GB; Tulshian, DB 2-(2-Furanyl)-7-phenyl[1,2,4]triazolo[1,5-c]pyrimidin-5-amine analogs: highly potent, orally active, adenosine A2A antagonists. Part 1. Bioorg Med Chem Lett15:3670-4 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | AA2AR_RAT | ADENOSINE A2a | Adenosine A2 receptor | Adenosine A2a receptor (A2a) | Adenosine Receptors A2a (A2a) | Adenosine receptor A2a and A3 | Adenosine receptors A2a | Adora2a | Rat striatal adenosine A2a receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 45015.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Rat A2A receptors expressed in CHO cells. |
|---|
| Residue: | 410 |
|---|
| Sequence: | MGSSVYITVELAIAVLAILGNVLVCWAVWINSNLQNVTNFFVVSLAAADIAVGVLAIPFA
ITISTGFCAACHGCLFFACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGVRAKG
IIAICWVLSFAIGLTPMLGWNNCSQKDGNSTKTCGEGRVTCLFEDVVPMNYMVYYNFFAF
VLLPLLLMLAIYLRIFLAARRQLKQMESQPLPGERTRSTLQKEVHAAKSLAIIVGLFALC
WLPLHIINCFTFFCSTCRHAPPWLMYLAIILSHSNSVVNPFIYAYRIREFRQTFRKIIRT
HVLRRQEPFQAGGSSAWALAAHSTEGEQVSLRLNGHPLGVWANGSATHSGRRPNGYTLGL
GGGGSAQGSPRDVELPTQERQEGQEHPGLRGHLVQARVGASSWSSEFAPS
|
|
|
|---|
| BDBM50169304 |
|---|
| n/a |
|---|
| Name | BDBM50169304 |
|---|
| Synonyms: | 2-Furan-2-yl-7-(3-{4-[3-methoxy-4-(2-methoxy-ethoxy)-phenyl]-piperazin-1-ylmethyl}-phenyl)-[1,2,4]triazolo[1,5-c]pyrimidin-5-ylamine | CHEMBL179167 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H33N7O4 |
|---|
| Mol. Mass. | 555.6275 |
|---|
| SMILES | COCCOc1ccc(cc1OC)N1CCN(Cc2cccc(c2)-c2cc3nc(nn3c(N)n2)-c2ccco2)CC1 |
|---|
| Structure | 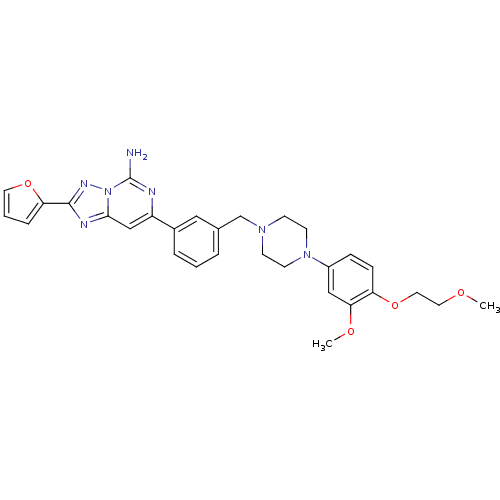
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Matasi, JJ; Caldwell, JP; Zhang, H; Fawzi, A; Cohen-Williams, ME; Varty, GB; Tulshian, DB 2-(2-Furanyl)-7-phenyl[1,2,4]triazolo[1,5-c]pyrimidin-5-amine analogs: highly potent, orally active, adenosine A2A antagonists. Part 1. Bioorg Med Chem Lett15:3670-4 (2005) [PubMed] Article
Matasi, JJ; Caldwell, JP; Zhang, H; Fawzi, A; Cohen-Williams, ME; Varty, GB; Tulshian, DB 2-(2-Furanyl)-7-phenyl[1,2,4]triazolo[1,5-c]pyrimidin-5-amine analogs: highly potent, orally active, adenosine A2A antagonists. Part 1. Bioorg Med Chem Lett15:3670-4 (2005) [PubMed] Article