

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202775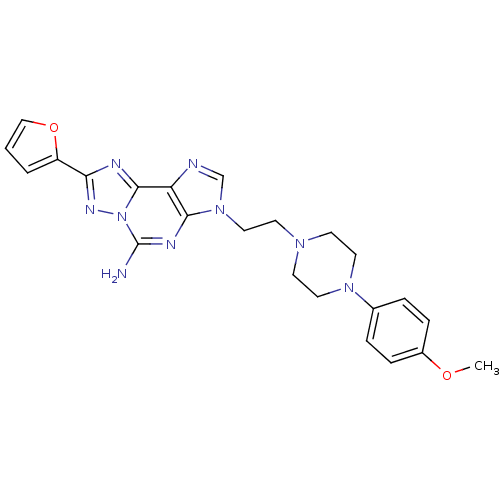 (8-(furan-2-yl)-3-(2-(4-(4-methoxyphenyl)piperazin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242266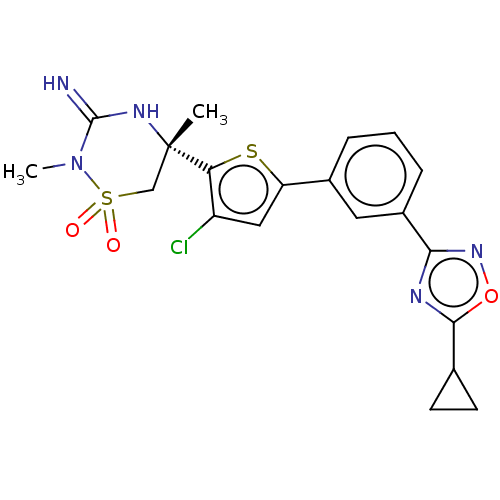 (US9416129, 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.240 | -55.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242250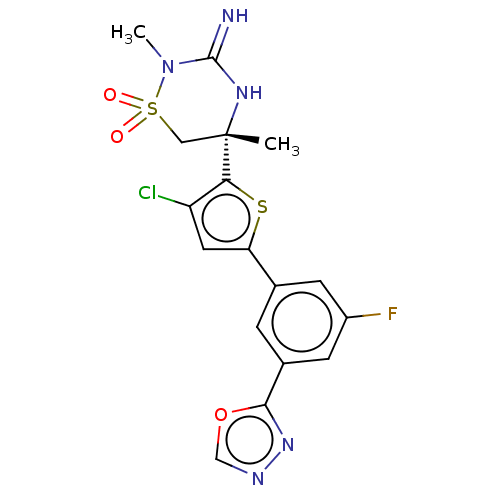 (US9416129, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.280 | -55.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50258880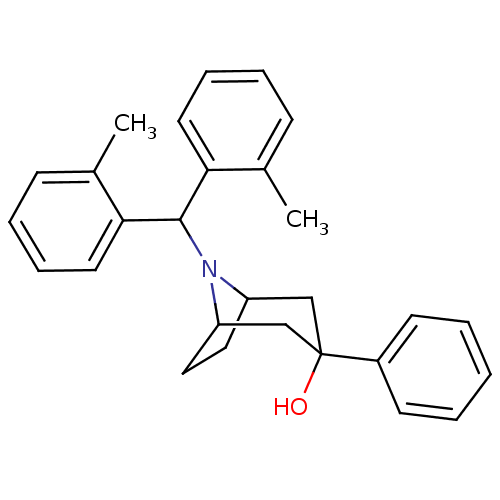 (8-(dio-tolylmethyl)-3-phenyl-8-azabicyclo[3.2.1]oc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human cloned NOP receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 2519-23 (2009) Article DOI: 10.1016/j.bmcl.2009.03.031 BindingDB Entry DOI: 10.7270/Q20V8CPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50204794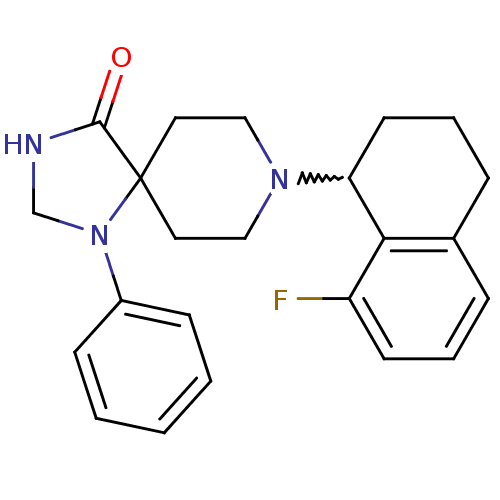 (8-(8-fluoro-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202777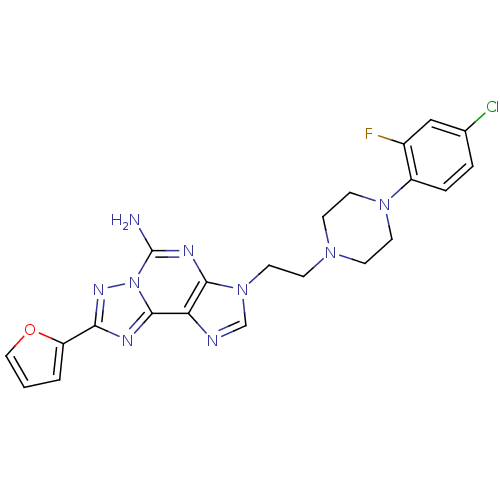 (3-(2-(4-(4-chloro-2-fluorophenyl)piperazin-1-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242231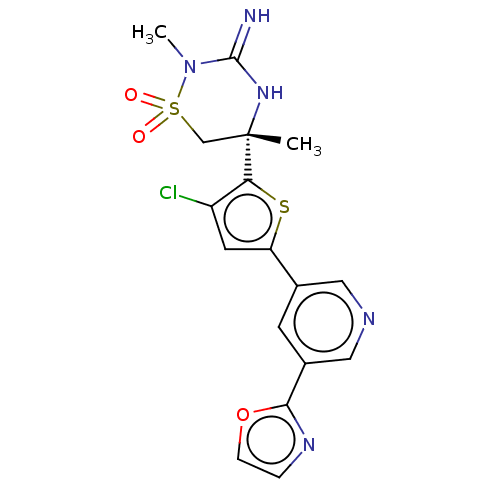 (US9416129, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.310 | -55.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242269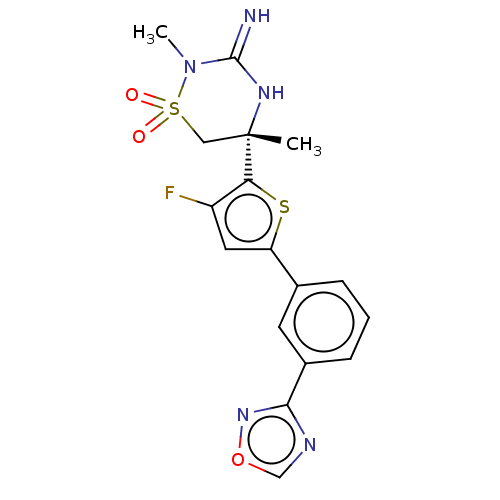 (US9416129, 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | -54.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242263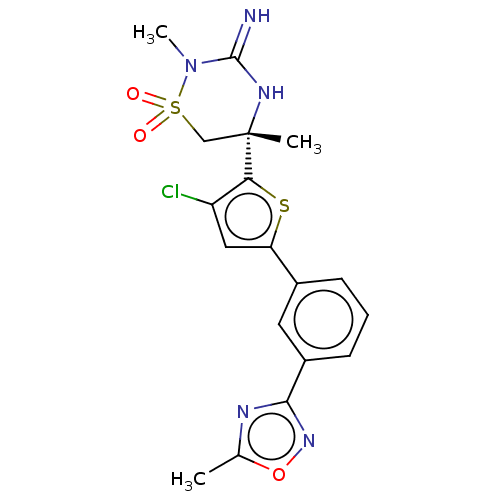 (US9416129, 41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | -54.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242226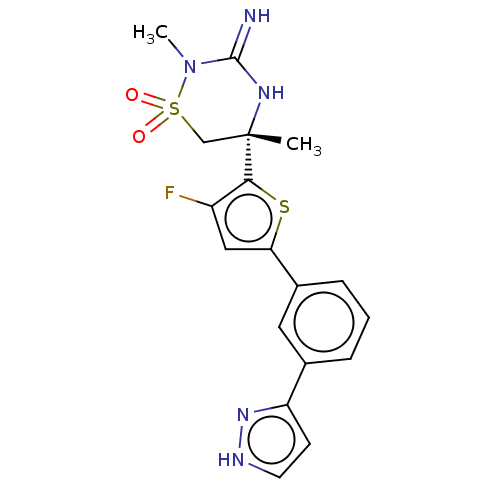 (US9416129, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | -54.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202771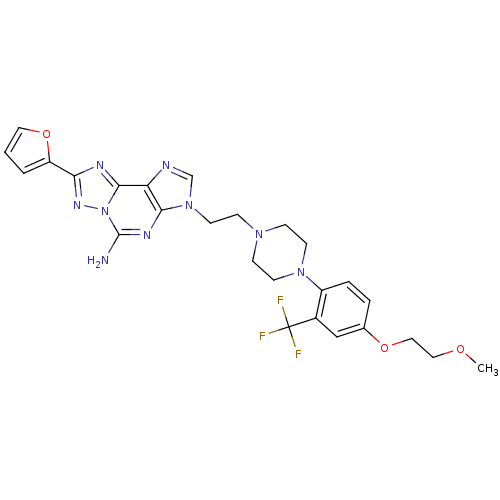 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)-2-(tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26927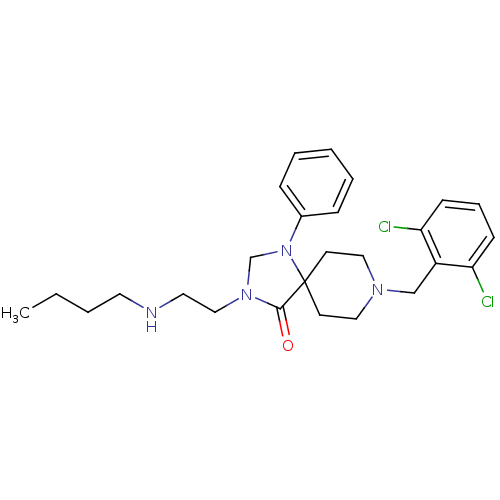 (3-[2-(butylamino)ethyl]-8-[(2,6-dichlorophenyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26946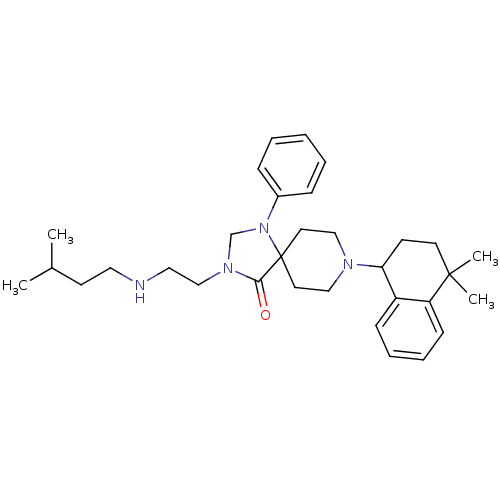 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202788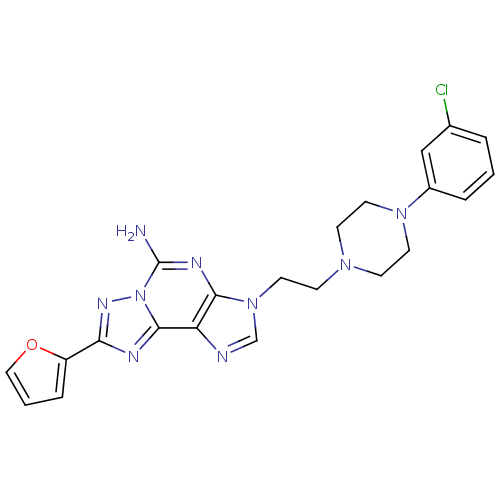 (3-(2-(4-(3-chlorophenyl)piperazin-1-yl)ethyl)-8-(f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242268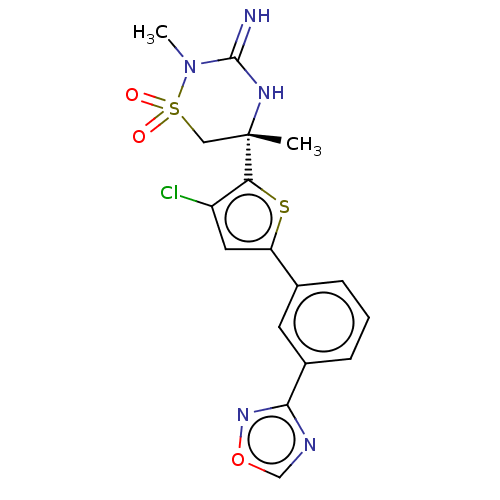 (US9416129, 46) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.410 | -54.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26942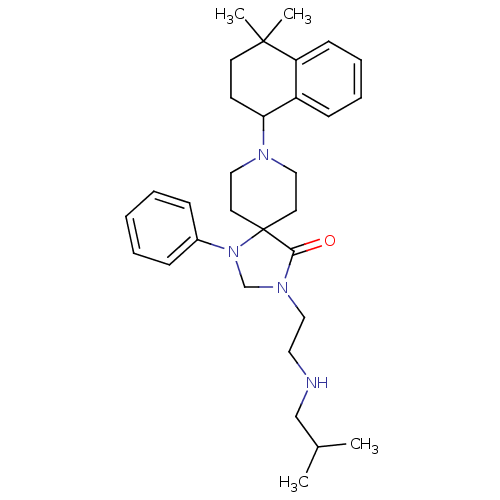 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26945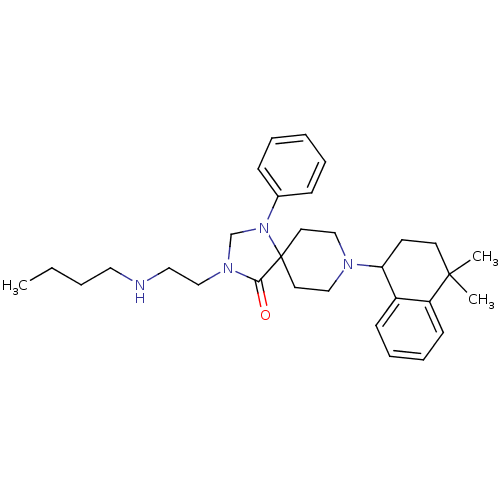 (3-[2-(butylamino)ethyl]-8-(4,4-dimethyl-1,2,3,4-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242250 (US9416129, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26923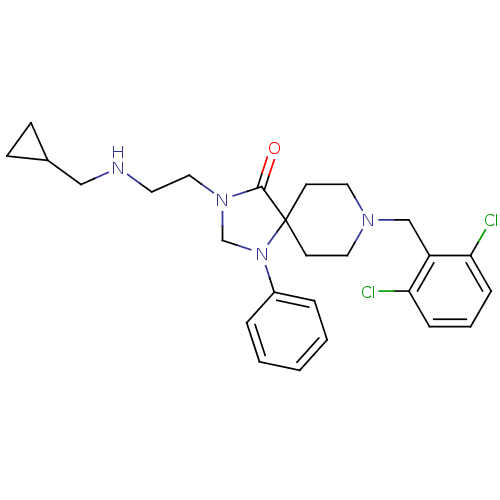 (3-{2-[(cyclopropylmethyl)amino]ethyl}-8-[(2,6-dich...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26926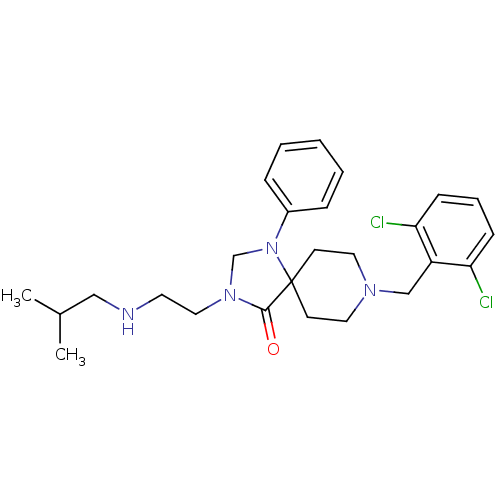 (8-[(2,6-dichlorophenyl)methyl]-3-{2-[(2-methylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26924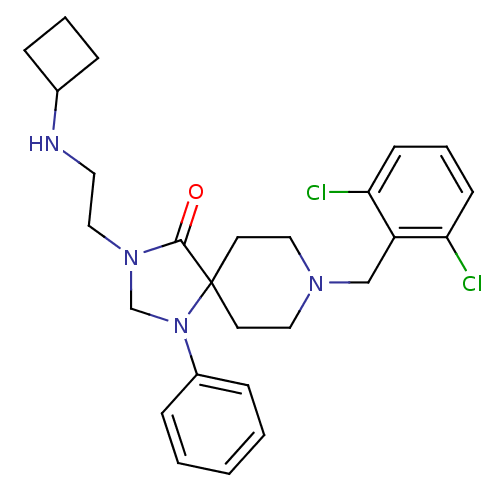 (3-[2-(cyclobutylamino)ethyl]-8-[(2,6-dichloropheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26954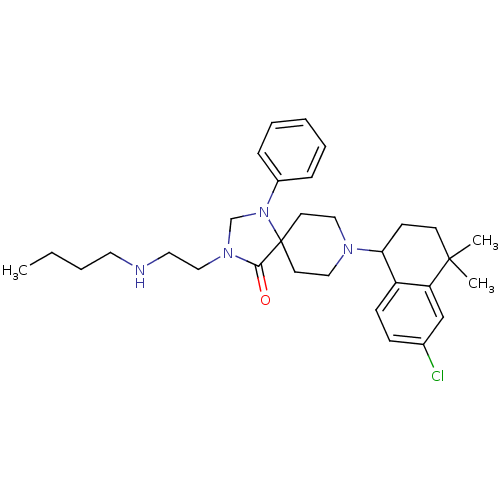 (3-[2-(butylamino)ethyl]-8-(6-chloro-4,4-dimethyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26925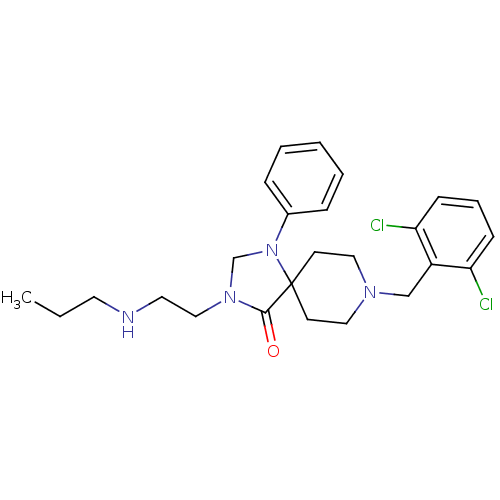 (8-[(2,6-dichlorophenyl)methyl]-1-phenyl-3-[2-(prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50065428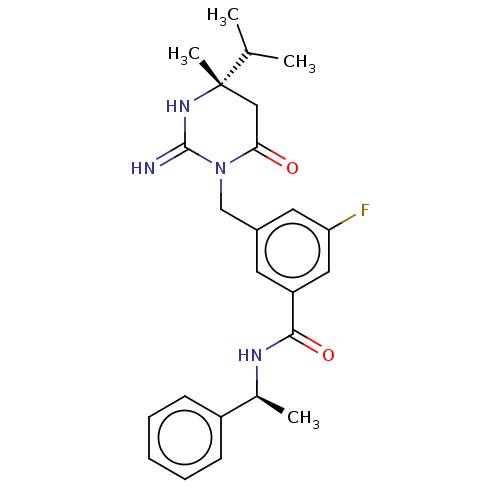 (CHEMBL3401350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | Bioorg Med Chem Lett 25: 1592-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.003 BindingDB Entry DOI: 10.7270/Q2X63PMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242270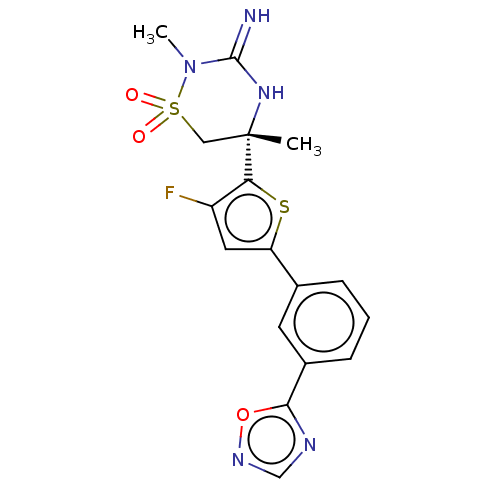 (US9416129, 48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | -53.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26950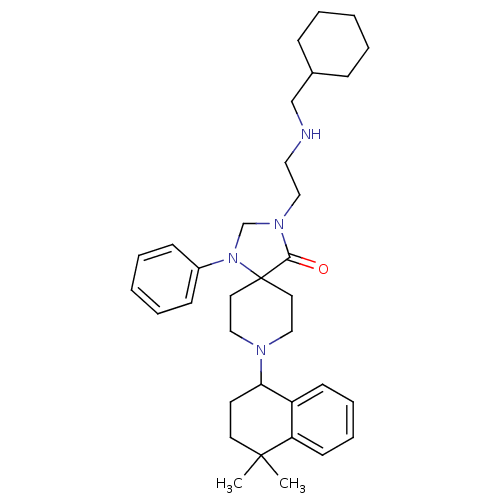 (3-{2-[(cyclohexylmethyl)amino]ethyl}-8-(4,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26922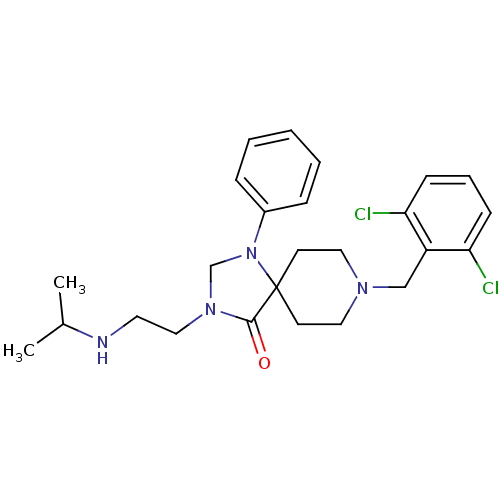 (8-[(2,6-dichlorophenyl)methyl]-1-phenyl-3-[2-(prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468037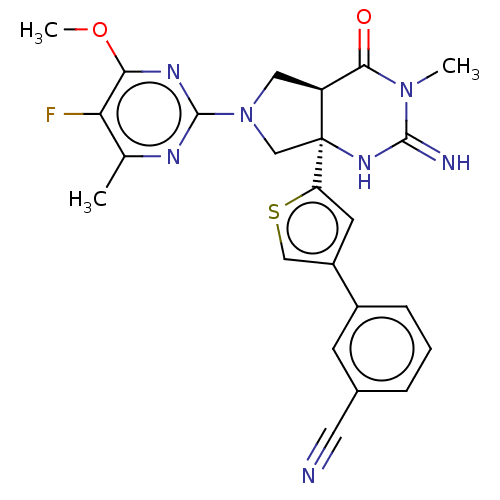 (CHEMBL4293298) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26921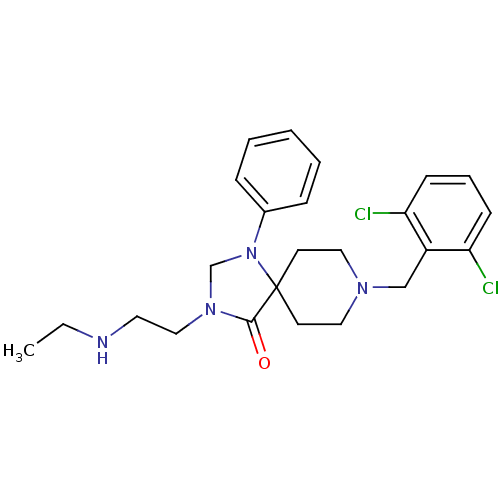 (8-[(2,6-dichlorophenyl)methyl]-3-[2-(ethylamino)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468040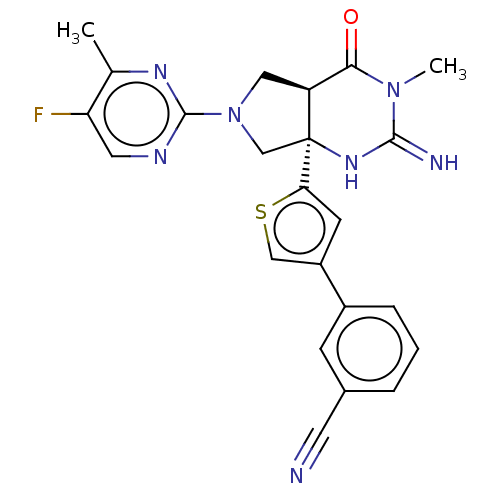 (CHEMBL4289763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202790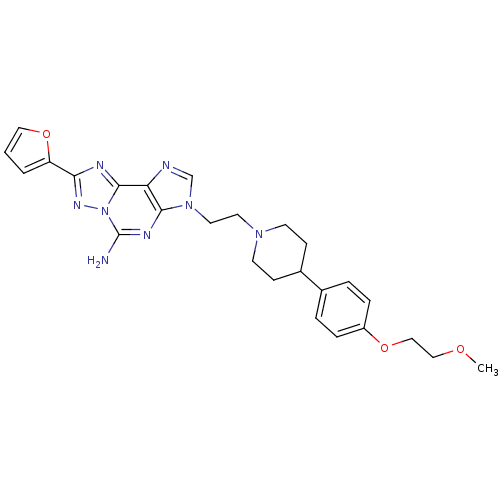 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202779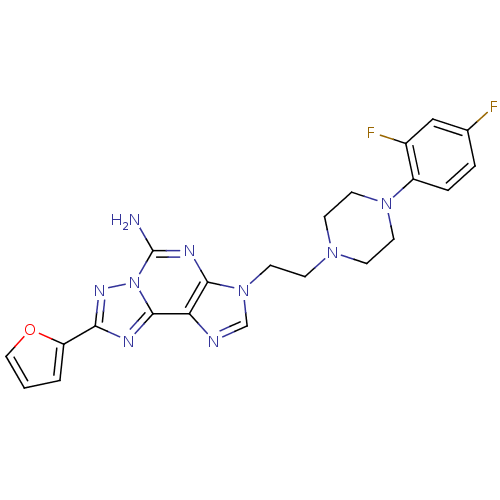 (3-(2-(4-(2,4-difluorophenyl)piperazin-1-yl)ethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM242229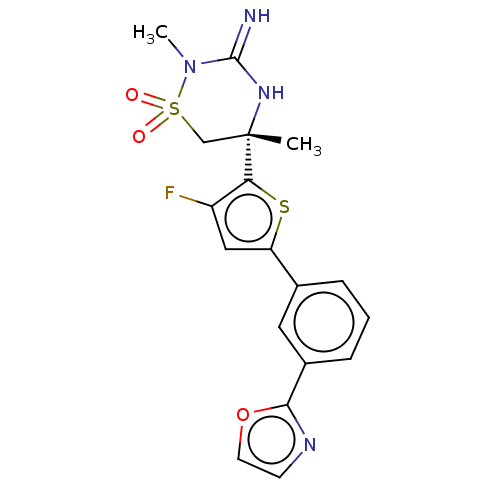 (US9416129, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.730 | -53.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibitor compounds, prepared at 3× the desired final concentration in 1×BACE assay buffer (20 mM sodium acetate pH 5.0, 10% glycerol, 0.... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26941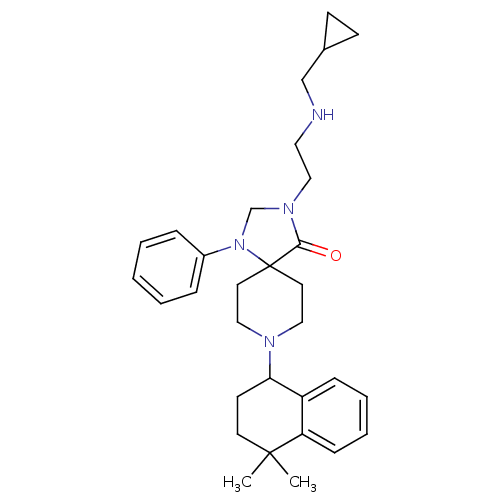 (3-{2-[(cyclopropylmethyl)amino]ethyl}-8-(4,4-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26920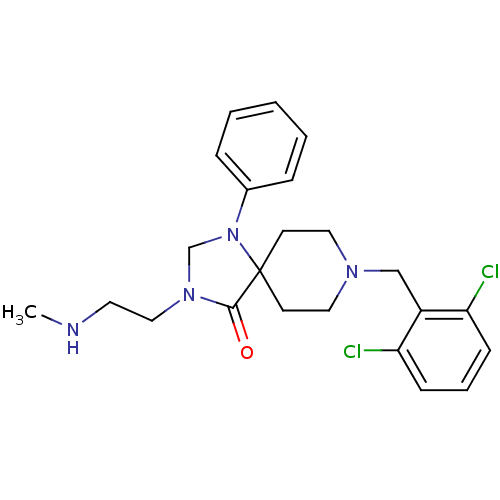 (8-[(2,6-dichlorophenyl)methyl]-3-[2-(methylamino)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202769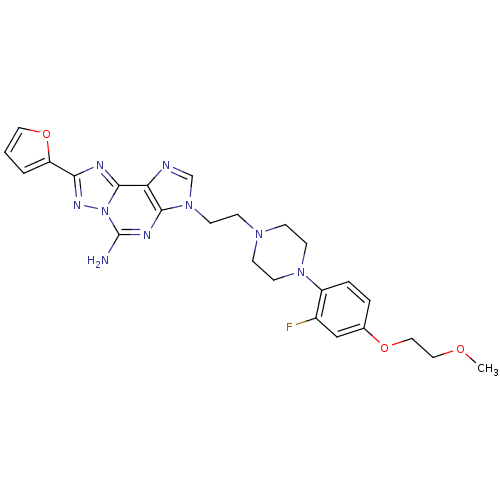 (3-(2-(4-(2-fluoro-4-(2-methoxyethoxy)phenyl)pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50204769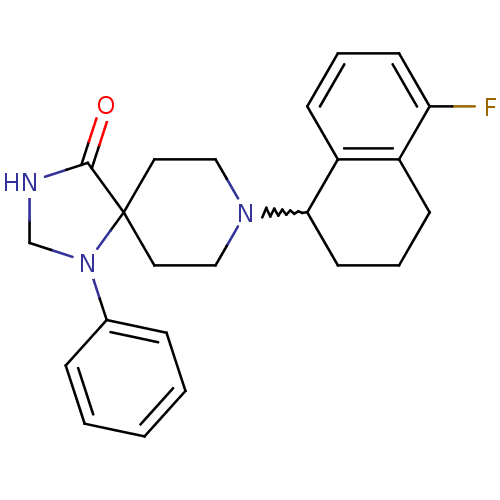 (8-(5-fluoro-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM242270 (US9416129, 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.870 | -52.6 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Varying concentrations of inhibitors at 3× the final desired concentration in a volume of 10 μl are preincubated with purified human BACE1 c... | US Patent US9416129 (2016) BindingDB Entry DOI: 10.7270/Q2HM57C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202783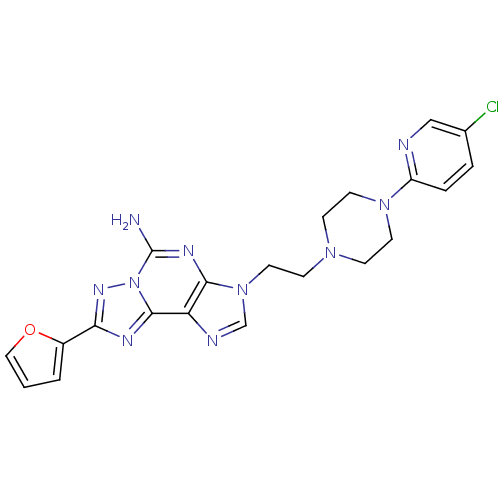 (3-(2-(4-(5-chloropyridin-2-yl)piperazin-1-yl)ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202773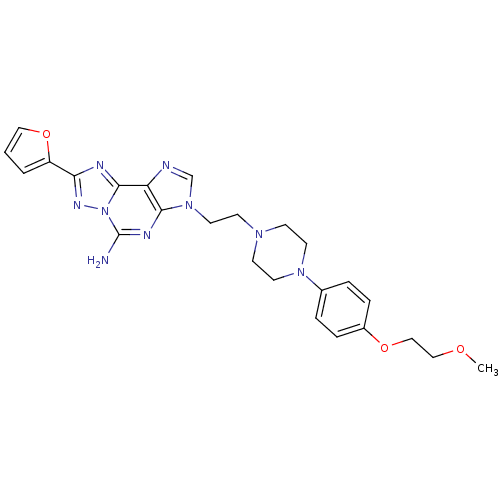 (8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26936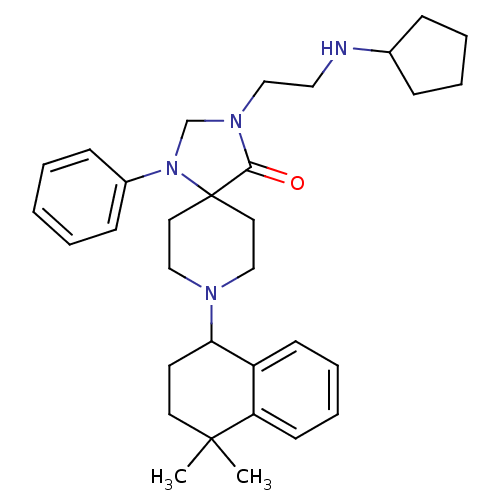 (3-[2-(cyclopentylamino)ethyl]-8-(4,4-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50204768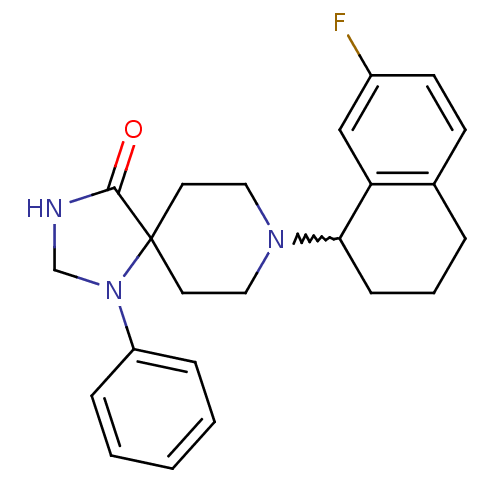 (8-(7-fluoro-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]nociceptin from human nociceptin receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 17: 2281-4 (2007) Article DOI: 10.1016/j.bmcl.2007.01.069 BindingDB Entry DOI: 10.7270/Q2T43SRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202766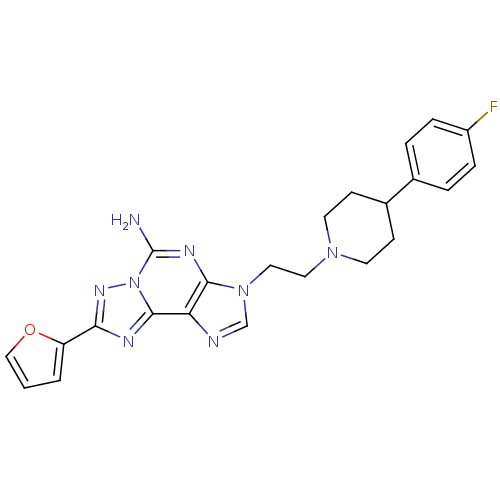 (3-(2-(4-(4-fluorophenyl)piperidin-1-yl)ethyl)-8-(f...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26937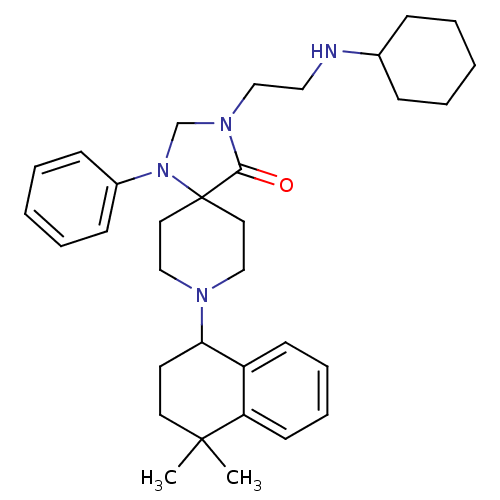 (3-[2-(cyclohexylamino)ethyl]-8-(4,4-dimethyl-1,2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26939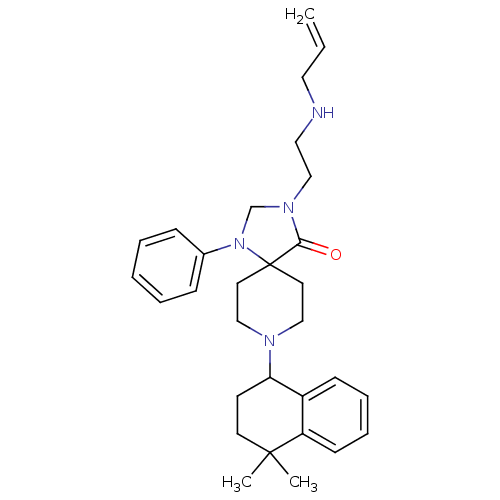 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50468043 (CHEMBL4288838) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398688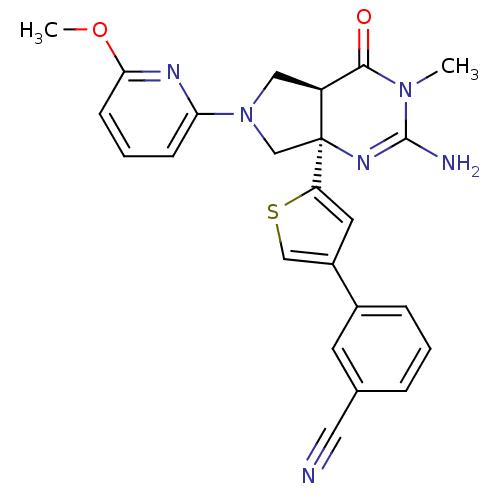 (CHEMBL2178715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 | J Med Chem 55: 9331-45 (2012) Article DOI: 10.1021/jm301039c BindingDB Entry DOI: 10.7270/Q2JH3NB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202767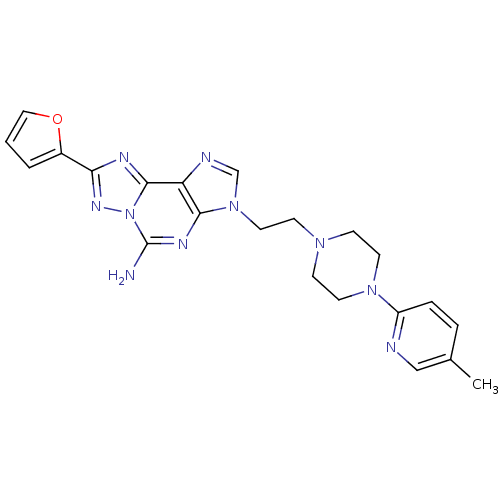 (8-(furan-2-yl)-3-(2-(4-(5-methylpyridin-2-yl)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor | Bioorg Med Chem Lett 17: 1659-62 (2007) Article DOI: 10.1016/j.bmcl.2006.12.104 BindingDB Entry DOI: 10.7270/Q2RF5TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50164512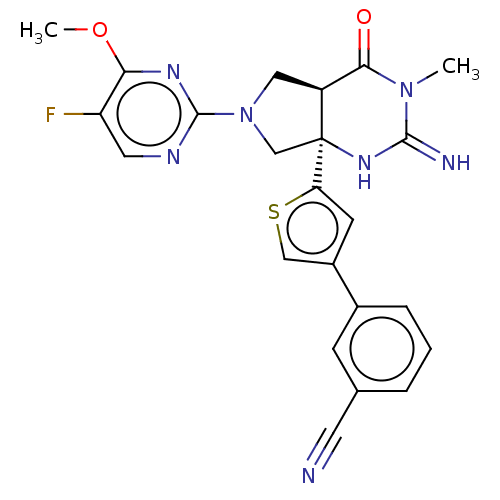 (CHEMBL3800286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 expressed in HEK293 cells | J Med Chem 61: 10700-10708 (2018) Article DOI: 10.1021/acs.jmedchem.8b01326 BindingDB Entry DOI: 10.7270/Q2VX0K6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26938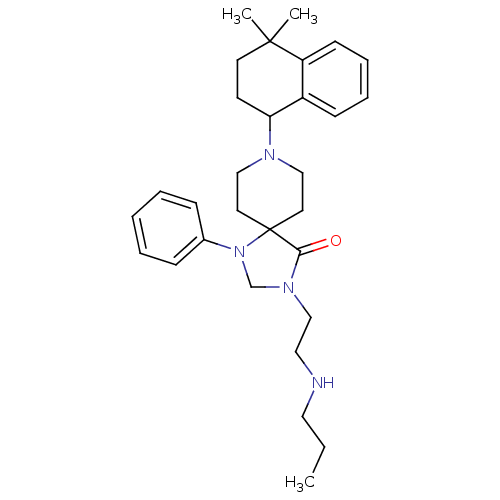 (8-(4,4-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1326 total ) | Next | Last >> |