| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peptidyl-prolyl cis-trans isomerase FKBP1A |
|---|
| Ligand | BDBM50606717 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2261184 (CHEMBL5216195) |
|---|
| Ki | 3.9±n/a nM |
|---|
| Citation |  Oleksak, P; Nepovimova, E; Chrienova, Z; Musilek, K; Patocka, J; Kuca, K Contemporary mTOR inhibitor scaffolds to diseases breakdown: A patent review (2015-2021). Eur J Med Chem238:0 (2022) [PubMed] Article Oleksak, P; Nepovimova, E; Chrienova, Z; Musilek, K; Patocka, J; Kuca, K Contemporary mTOR inhibitor scaffolds to diseases breakdown: A patent review (2015-2021). Eur J Med Chem238:0 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peptidyl-prolyl cis-trans isomerase FKBP1A |
|---|
| Name: | Peptidyl-prolyl cis-trans isomerase FKBP1A |
|---|
| Synonyms: | 12 kDa FK506-binding protein | 12 kDa FKBP | FK506-binding protein 1A | FK506-binding protein 1A (FKBP12) | FKB1A_HUMAN | FKBP-12 | FKBP-1A | FKBP1 | FKBP12 | FKBP1A | Immunophilin FKBP12 | PPIase | PPIase FKBP1A | Peptidyl-prolyl cis-trans isomerase (FKBP) | Rotamase | RyR1/FKBP12 | mTOR/FKBP12A/FKBP12B |
|---|
| Type: | Isomerase |
|---|
| Mol. Mass.: | 11953.09 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P62942 |
|---|
| Residue: | 108 |
|---|
| Sequence: | MGVQVETISPGDGRTFPKRGQTCVVHYTGMLEDGKKFDSSRDRNKPFKFMLGKQEVIRGW
EEGVAQMSVGQRAKLTISPDYAYGATGHPGIIPPHATLVFDVELLKLE
|
|
|
|---|
| BDBM50606717 |
|---|
| n/a |
|---|
| Name | BDBM50606717 |
|---|
| Synonyms: | CHEMBL5218737 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C51H77NO12 |
|---|
| Mol. Mass. | 896.1566 |
|---|
| SMILES | [H][C@]12C[C@@H](C[C@@H](C)C3CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@]4([H])CC[C@@H](C)[C@@](O)(O4)C(=O)C(=O)N4CCCC[C@H]4C(=O)O3)OC)[C@]([H])(C[C@H]1O)C2 |r,c:13,32,t:28,30| |
|---|
| Structure | 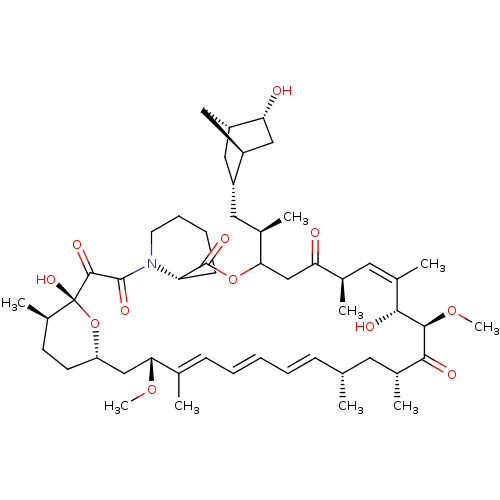
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Oleksak, P; Nepovimova, E; Chrienova, Z; Musilek, K; Patocka, J; Kuca, K Contemporary mTOR inhibitor scaffolds to diseases breakdown: A patent review (2015-2021). Eur J Med Chem238:0 (2022) [PubMed] Article
Oleksak, P; Nepovimova, E; Chrienova, Z; Musilek, K; Patocka, J; Kuca, K Contemporary mTOR inhibitor scaffolds to diseases breakdown: A patent review (2015-2021). Eur J Med Chem238:0 (2022) [PubMed] Article