| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50220012 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_445969 (CHEMBL896268) |
|---|
| IC50 | >20000±n/a nM |
|---|
| Citation |  Reck, F; Zhou, F; Eyermann, CJ; Kern, G; Carcanague, D; Ioannidis, G; Illingworth, R; Poon, G; Gravestock, MB Novel substituted (pyridin-3-yl)phenyloxazolidinones: antibacterial agents with reduced activity against monoamine oxidase A and increased solubility. J Med Chem50:4868-81 (2007) [PubMed] Article Reck, F; Zhou, F; Eyermann, CJ; Kern, G; Carcanague, D; Ioannidis, G; Illingworth, R; Poon, G; Gravestock, MB Novel substituted (pyridin-3-yl)phenyloxazolidinones: antibacterial agents with reduced activity against monoamine oxidase A and increased solubility. J Med Chem50:4868-81 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50220012 |
|---|
| n/a |
|---|
| Name | BDBM50220012 |
|---|
| Synonyms: | (5R)-3-(3-fluoro-4-pyridin-3-ylphenyl)-5-(1H-1,2,3-triazol-1-ylmethyl)-1,3-oxazolidin-2-one | CHEMBL394864 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H14FN5O2 |
|---|
| Mol. Mass. | 339.3238 |
|---|
| SMILES | Fc1cc(ccc1-c1cccnc1)N1C[C@H](Cn2ccnn2)OC1=O |
|---|
| Structure | 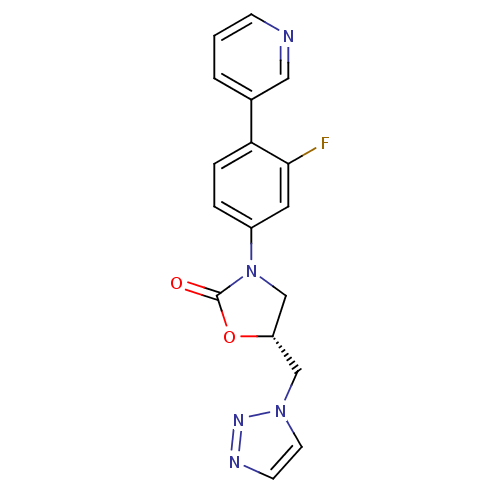
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Reck, F; Zhou, F; Eyermann, CJ; Kern, G; Carcanague, D; Ioannidis, G; Illingworth, R; Poon, G; Gravestock, MB Novel substituted (pyridin-3-yl)phenyloxazolidinones: antibacterial agents with reduced activity against monoamine oxidase A and increased solubility. J Med Chem50:4868-81 (2007) [PubMed] Article
Reck, F; Zhou, F; Eyermann, CJ; Kern, G; Carcanague, D; Ioannidis, G; Illingworth, R; Poon, G; Gravestock, MB Novel substituted (pyridin-3-yl)phenyloxazolidinones: antibacterial agents with reduced activity against monoamine oxidase A and increased solubility. J Med Chem50:4868-81 (2007) [PubMed] Article