| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50332229 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_688019 (CHEMBL1291743) |
|---|
| IC50 | >40000±n/a nM |
|---|
| Citation |  Nishio, Y; Kimura, H; Tosaki, S; Sugaru, E; Sakai, M; Horiguchi, M; Masui, Y; Ono, M; Nakagawa, T; Nakahira, H Discovery of new chemotype dipeptidyl peptidase IV inhibitors having (R)-3-amino-3-methyl piperidine as a pharmacophore. Bioorg Med Chem Lett20:7246-9 (2010) [PubMed] Article Nishio, Y; Kimura, H; Tosaki, S; Sugaru, E; Sakai, M; Horiguchi, M; Masui, Y; Ono, M; Nakagawa, T; Nakahira, H Discovery of new chemotype dipeptidyl peptidase IV inhibitors having (R)-3-amino-3-methyl piperidine as a pharmacophore. Bioorg Med Chem Lett20:7246-9 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50332229 |
|---|
| n/a |
|---|
| Name | BDBM50332229 |
|---|
| Synonyms: | (R)-6-(3-amino-3-methylpiperidin-1-yl)-5-(5-fluoro-2-methylbenzyl)-3-methyl-3H-pyrrolo[3,2-d]pyrimidin-4(5H)-one hydrochloride | CHEMBL1287901 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H26FN5O |
|---|
| Mol. Mass. | 383.4624 |
|---|
| SMILES | Cc1ccc(F)cc1Cn1c(cc2ncn(C)c(=O)c12)N1CCC[C@@](C)(N)C1 |r| |
|---|
| Structure | 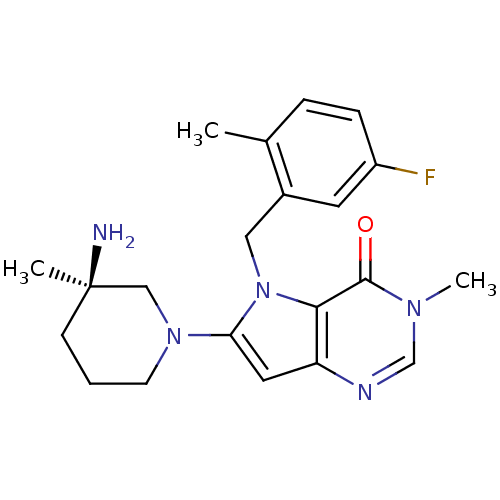
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nishio, Y; Kimura, H; Tosaki, S; Sugaru, E; Sakai, M; Horiguchi, M; Masui, Y; Ono, M; Nakagawa, T; Nakahira, H Discovery of new chemotype dipeptidyl peptidase IV inhibitors having (R)-3-amino-3-methyl piperidine as a pharmacophore. Bioorg Med Chem Lett20:7246-9 (2010) [PubMed] Article
Nishio, Y; Kimura, H; Tosaki, S; Sugaru, E; Sakai, M; Horiguchi, M; Masui, Y; Ono, M; Nakagawa, T; Nakahira, H Discovery of new chemotype dipeptidyl peptidase IV inhibitors having (R)-3-amino-3-methyl piperidine as a pharmacophore. Bioorg Med Chem Lett20:7246-9 (2010) [PubMed] Article