| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Bifunctional epoxide hydrolase 2 |
|---|
| Ligand | BDBM50435171 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_963496 (CHEMBL2394377) |
|---|
| IC50 | 1498±n/a nM |
|---|
| Citation |  North, EJ; Scherman, MS; Bruhn, DF; Scarborough, JS; Maddox, MM; Jones, V; Grzegorzewicz, A; Yang, L; Hess, T; Morisseau, C; Jackson, M; McNeil, MR; Lee, RE Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorg Med Chem21:2587-99 (2013) [PubMed] Article North, EJ; Scherman, MS; Bruhn, DF; Scarborough, JS; Maddox, MM; Jones, V; Grzegorzewicz, A; Yang, L; Hess, T; Morisseau, C; Jackson, M; McNeil, MR; Lee, RE Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorg Med Chem21:2587-99 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Bifunctional epoxide hydrolase 2 |
|---|
| Name: | Bifunctional epoxide hydrolase 2 |
|---|
| Synonyms: | Cytosolic epoxide hydrolase 2 | EBifunctional epoxide hydrolase 2 | EPHX2 | Epoxide hydratase | HYES_HUMAN | Lipid-phosphate phosphatase | Soluble epoxide hydrolase (sEH) | epoxide hydrolase 2, cytoplasmic |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 62613.07 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P34913 |
|---|
| Residue: | 555 |
|---|
| Sequence: | MTLRAAVFDLDGVLALPAVFGVLGRTEEALALPRGLLNDAFQKGGPEGATTRLMKGEITL
SQWIPLMEENCRKCSETAKVCLPKNFSIKEIFDKAISARKINRPMLQAALMLRKKGFTTA
ILTNTWLDDRAERDGLAQLMCELKMHFDFLIESCQVGMVKPEPQIYKFLLDTLKASPSEV
VFLDDIGANLKPARDLGMVTILVQDTDTALKELEKVTGIQLLNTPAPLPTSCNPSDMSHG
YVTVKPRVRLHFVELGSGPAVCLCHGFPESWYSWRYQIPALAQAGYRVLAMDMKGYGESS
APPEIEEYCMEVLCKEMVTFLDKLGLSQAVFIGHDWGGMLVWYMALFYPERVRAVASLNT
PFIPANPNMSPLESIKANPVFDYQLYFQEPGVAEAELEQNLSRTFKSLFRASDESVLSMH
KVCEAGGLFVNSPEEPSLSRMVTEEEIQFYVQQFKKSGFRGPLNWYRNMERNWKWACKSL
GRKILIPALMVTAEKDFVLVPQMSQHMEDWIPHLKRGHIEDCGHWTQMDKPTEVNQILIK
WLDSDARNPPVVSKM
|
|
|
|---|
| BDBM50435171 |
|---|
| n/a |
|---|
| Name | BDBM50435171 |
|---|
| Synonyms: | CHEMBL1346880 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H21N3O |
|---|
| Mol. Mass. | 271.3574 |
|---|
| SMILES | O=C(Nc1ccccn1)NC12CC3CC(CC(C3)C1)C2 |TLB:13:14:18:12.11.17,THB:13:12:18:14.19.15,15:14:11:16.18.17,15:16:11:14.19.13| |
|---|
| Structure | 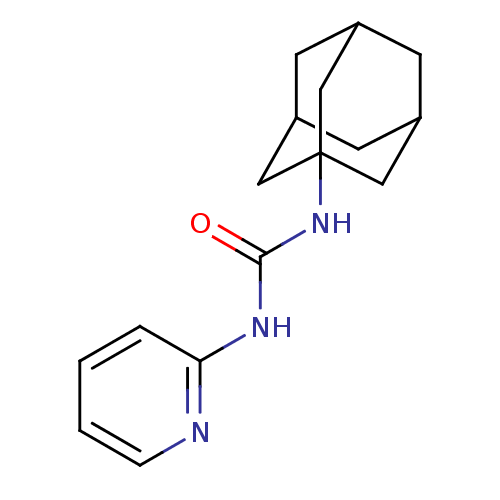
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 North, EJ; Scherman, MS; Bruhn, DF; Scarborough, JS; Maddox, MM; Jones, V; Grzegorzewicz, A; Yang, L; Hess, T; Morisseau, C; Jackson, M; McNeil, MR; Lee, RE Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorg Med Chem21:2587-99 (2013) [PubMed] Article
North, EJ; Scherman, MS; Bruhn, DF; Scarborough, JS; Maddox, MM; Jones, V; Grzegorzewicz, A; Yang, L; Hess, T; Morisseau, C; Jackson, M; McNeil, MR; Lee, RE Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorg Med Chem21:2587-99 (2013) [PubMed] Article