 Found 13298 hits with Last Name = 'yang' and Initial = 'l'
Found 13298 hits with Last Name = 'yang' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85357
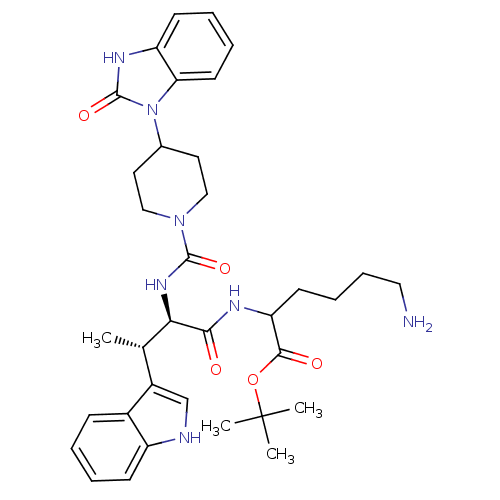
(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102777
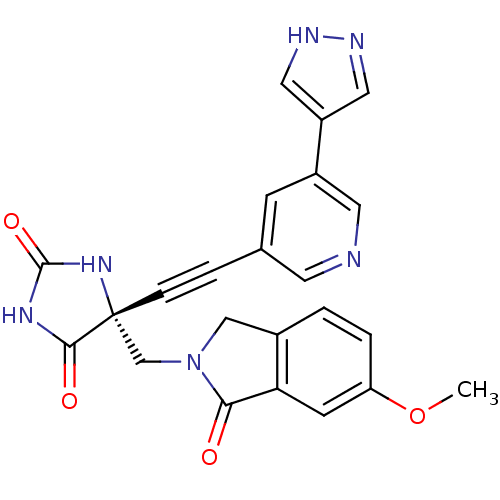
(US8541572, 2207)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cncc(c3)-c3cn[nH]c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N6O4/c1-33-18-3-2-15-12-29(20(30)19(15)7-18)13-23(21(31)27-22(32)28-23)5-4-14-6-16(9-24-8-14)17-10-25-26-11-17/h2-3,6-11H,12-13H2,1H3,(H,25,26)(H2,27,28,31,32)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102624
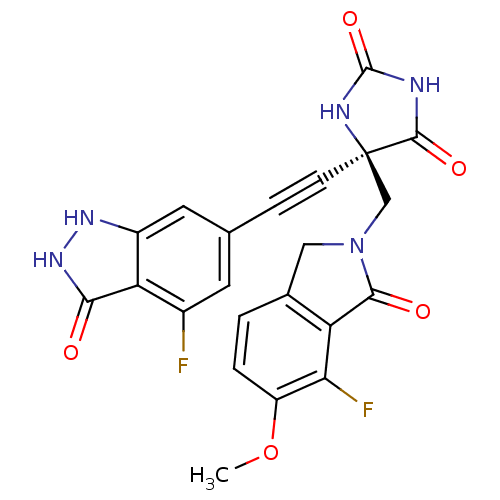
(US8541572, 303)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cc(F)c4c(c3)[nH][nH]c4=O)C(=O)c2c1F |r| Show InChI InChI=1S/C22H15F2N5O5/c1-34-14-3-2-11-8-29(19(31)15(11)17(14)24)9-22(20(32)25-21(33)26-22)5-4-10-6-12(23)16-13(7-10)27-28-18(16)30/h2-3,6-7H,8-9H2,1H3,(H2,27,28,30)(H2,25,26,32,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | 383 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064772
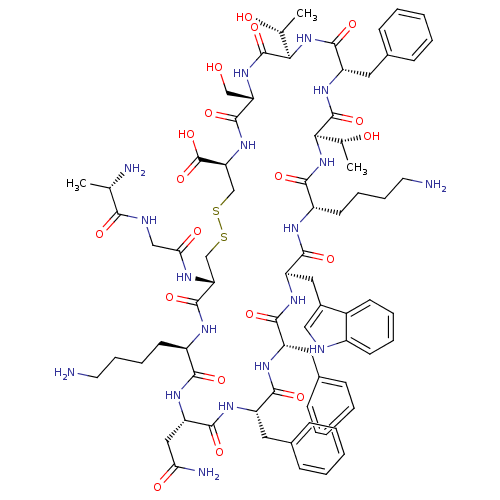
(Ala-Gly-cyclo[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52-,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human sst2 receptor expressed in CHO-K1 cells |
J Med Chem 41: 2175-9 (1998)
Article DOI: 10.1021/jm980194h
BindingDB Entry DOI: 10.7270/Q2XW4KGS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50513202
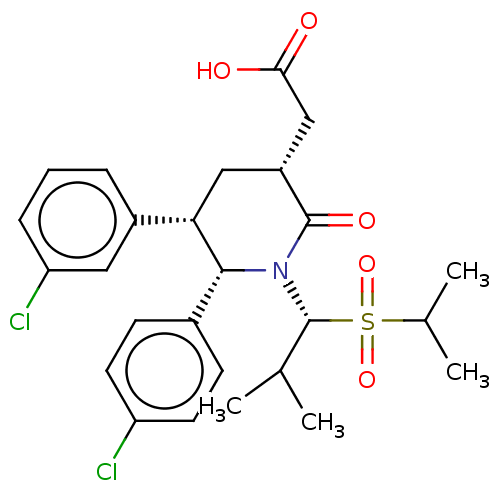
(CHEMBL4463050)Show SMILES CC(C)[C@H](N1[C@@H]([C@@H](C[C@H](CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)S(=O)(=O)C(C)C |r| Show InChI InChI=1S/C26H31Cl2NO5S/c1-15(2)26(35(33,34)16(3)4)29-24(17-8-10-20(27)11-9-17)22(18-6-5-7-21(28)12-18)13-19(25(29)32)14-23(30)31/h5-12,15-16,19,22,24,26H,13-14H2,1-4H3,(H,30,31)/t19-,22+,24-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of p53 protein binding to MDM2 in human SJSA1 cells |
Eur J Med Chem 176: 92-104 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.018
BindingDB Entry DOI: 10.7270/Q2V12851 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81766
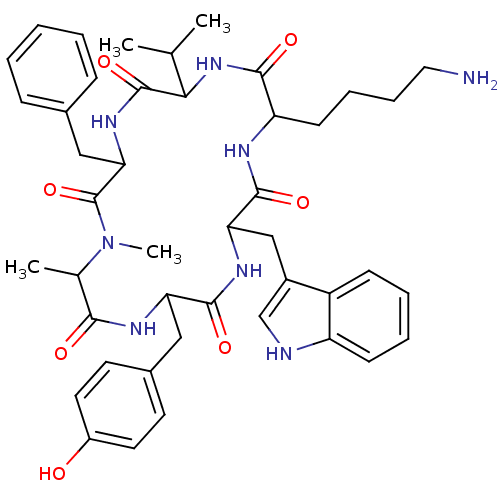
(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102623
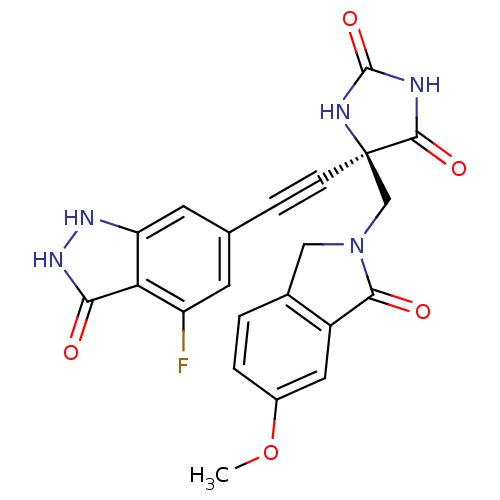
(US8541572, 302)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cc(F)c4c(c3)[nH][nH]c4=O)C(=O)c2c1 |r| Show InChI InChI=1S/C22H16FN5O5/c1-33-13-3-2-12-9-28(19(30)14(12)8-13)10-22(20(31)24-21(32)25-22)5-4-11-6-15(23)17-16(7-11)26-27-18(17)29/h2-3,6-8H,9-10H2,1H3,(H2,26,27,29)(H2,24,25,31,32)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0521 | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102666
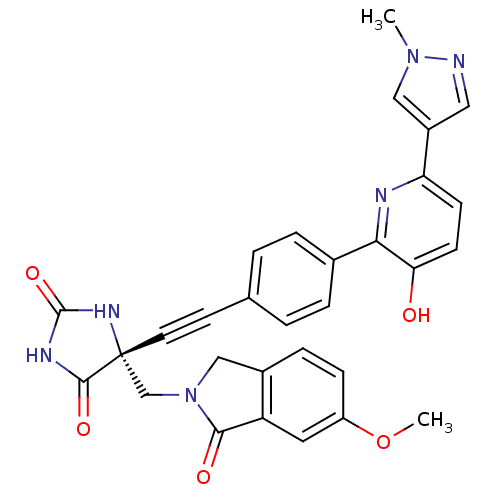
(US8541572, 973)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3nc(ccc3O)-c3cnn(C)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C30H24N6O5/c1-35-15-21(14-31-35)24-9-10-25(37)26(32-24)19-5-3-18(4-6-19)11-12-30(28(39)33-29(40)34-30)17-36-16-20-7-8-22(41-2)13-23(20)27(36)38/h3-10,13-15,37H,16-17H2,1-2H3,(H2,33,34,39,40)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0524 | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102663
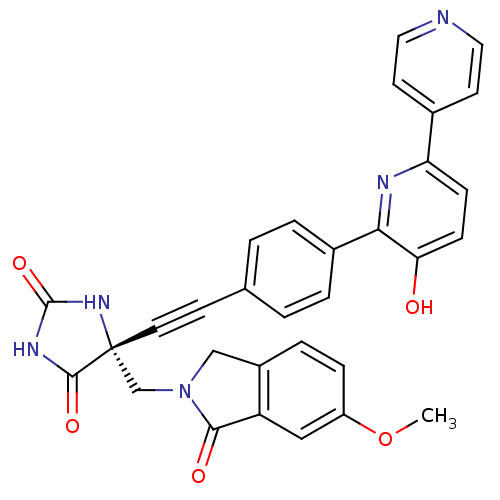
(US8541572, 970)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3nc(ccc3O)-c3ccncc3)C(=O)c2c1 |r| Show InChI InChI=1S/C31H23N5O5/c1-41-23-7-6-22-17-36(28(38)24(22)16-23)18-31(29(39)34-30(40)35-31)13-10-19-2-4-21(5-3-19)27-26(37)9-8-25(33-27)20-11-14-32-15-12-20/h2-9,11-12,14-16,37H,17-18H2,1H3,(H2,34,35,39,40)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0556 | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102802
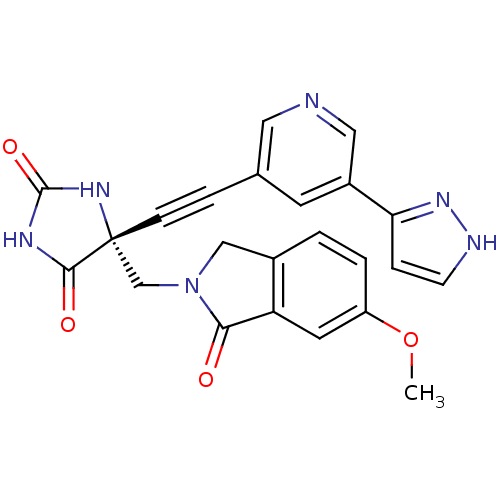
(US8541572, 2234)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cncc(c3)-c3cc[nH]n3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N6O4/c1-33-17-3-2-15-12-29(20(30)18(15)9-17)13-23(21(31)26-22(32)27-23)6-4-14-8-16(11-24-10-14)19-5-7-25-28-19/h2-3,5,7-11H,12-13H2,1H3,(H,25,28)(H2,26,27,31,32)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0600 | n/a | 678 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332270
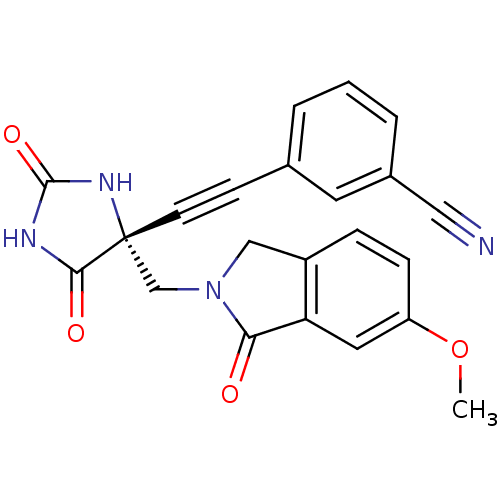
((R)-3-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(c3)C#N)C(=O)c2c1 |r| Show InChI InChI=1S/C22H16N4O4/c1-30-17-6-5-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)8-7-14-3-2-4-15(9-14)11-23/h2-6,9-10H,12-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102680
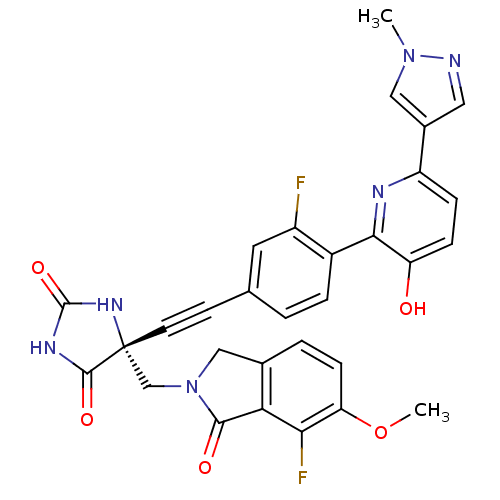
(US8541572, 987)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(c(F)c3)-c3nc(ccc3O)-c3cnn(C)c3)C(=O)c2c1F |r| Show InChI InChI=1S/C30H22F2N6O5/c1-37-13-18(12-33-37)21-6-7-22(39)26(34-21)19-5-3-16(11-20(19)31)9-10-30(28(41)35-29(42)36-30)15-38-14-17-4-8-23(43-2)25(32)24(17)27(38)40/h3-8,11-13,39H,14-15H2,1-2H3,(H2,35,36,41,42)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0627 | n/a | 75.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102845
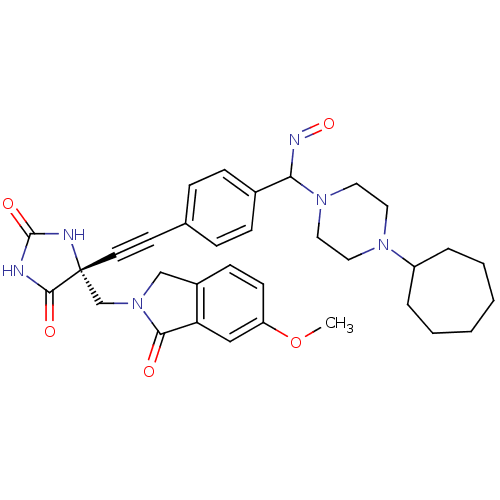
(US8541572, 927)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(N=O)N3CCN(CC3)C3CCCCCC3)C(=O)c2c1 |r| Show InChI InChI=1S/C33H38N6O5/c1-44-27-13-12-25-21-39(30(40)28(25)20-27)22-33(31(41)34-32(42)35-33)15-14-23-8-10-24(11-9-23)29(36-43)38-18-16-37(17-19-38)26-6-4-2-3-5-7-26/h8-13,20,26,29H,2-7,16-19,21-22H2,1H3,(H2,34,35,41,42)/t29?,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0630 | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102664

(US8541572, 971)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3nc(ccc3O)-c3cccnc3)C(=O)c2c1 |r| Show InChI InChI=1S/C31H23N5O5/c1-41-23-9-8-22-17-36(28(38)24(22)15-23)18-31(29(39)34-30(40)35-31)13-12-19-4-6-20(7-5-19)27-26(37)11-10-25(33-27)21-3-2-14-32-16-21/h2-11,14-16,37H,17-18H2,1H3,(H2,34,35,39,40)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0651 | n/a | 80.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332292
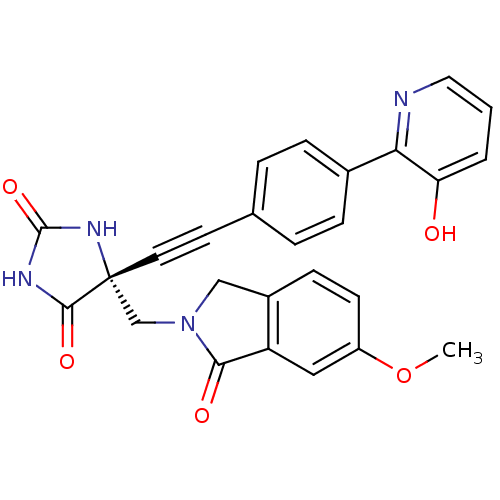
((R)-5-((4-(3-hydroxypyridin-2-yl)phenyl)ethynyl)-5...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3ncccc3O)C(=O)c2c1 |r| Show InChI InChI=1S/C26H20N4O5/c1-35-19-9-8-18-14-30(23(32)20(18)13-19)15-26(24(33)28-25(34)29-26)11-10-16-4-6-17(7-5-16)22-21(31)3-2-12-27-22/h2-9,12-13,31H,14-15H2,1H3,(H2,28,29,33,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102840
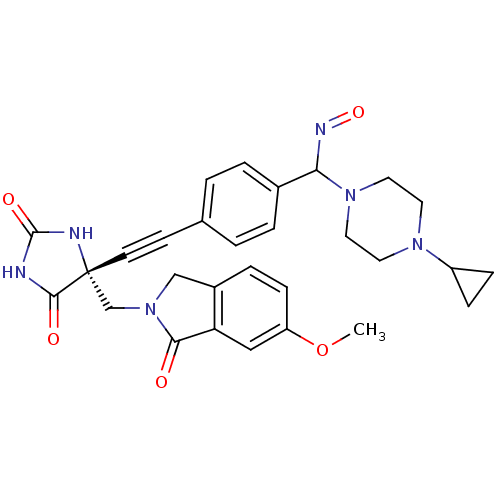
(US8541572, 922)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(N=O)N3CCN(CC3)C3CC3)C(=O)c2c1 |r| Show InChI InChI=1S/C29H30N6O5/c1-40-23-9-6-21-17-35(26(36)24(21)16-23)18-29(27(37)30-28(38)31-29)11-10-19-2-4-20(5-3-19)25(32-39)34-14-12-33(13-15-34)22-7-8-22/h2-6,9,16,22,25H,7-8,12-15,17-18H2,1H3,(H2,30,31,37,38)/t25?,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0720 | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50332292

((R)-5-((4-(3-hydroxypyridin-2-yl)phenyl)ethynyl)-5...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3ncccc3O)C(=O)c2c1 |r| Show InChI InChI=1S/C26H20N4O5/c1-35-19-9-8-18-14-30(23(32)20(18)13-19)15-26(24(33)28-25(34)29-26)11-10-16-4-6-17(7-5-16)22-21(31)3-2-12-27-22/h2-9,12-13,31H,14-15H2,1H3,(H2,28,29,33,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 0.0740 | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102698
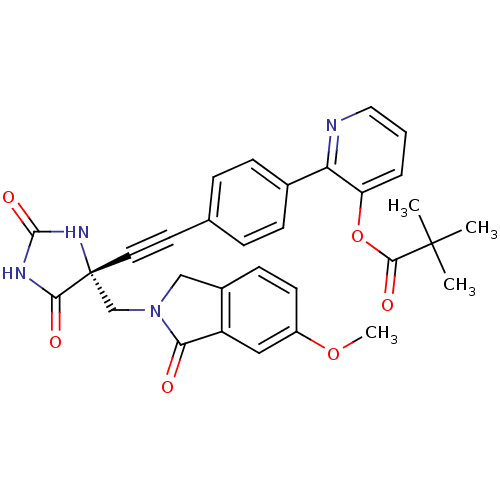
(US8541572, 1008)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3ncccc3OC(=O)C(C)(C)C)C(=O)c2c1 |r| Show InChI InChI=1S/C31H28N4O6/c1-30(2,3)28(38)41-24-6-5-15-32-25(24)20-9-7-19(8-10-20)13-14-31(27(37)33-29(39)34-31)18-35-17-21-11-12-22(40-4)16-23(21)26(35)36/h5-12,15-16H,17-18H2,1-4H3,(H2,33,34,37,39)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0762 | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102695
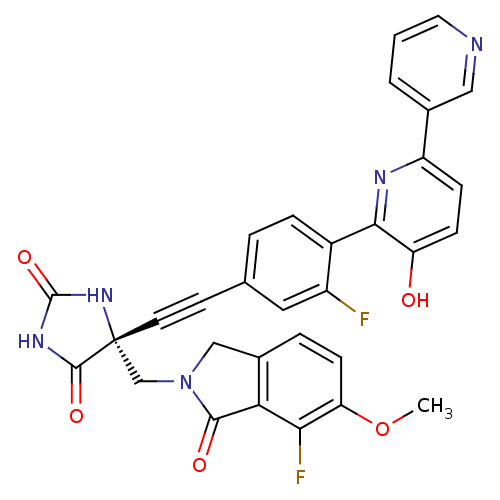
(US8541572, 1002)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(c(F)c3)-c3nc(ccc3O)-c3cccnc3)C(=O)c2c1F |r| Show InChI InChI=1S/C31H21F2N5O5/c1-43-24-9-5-19-15-38(28(40)25(19)26(24)33)16-31(29(41)36-30(42)37-31)11-10-17-4-6-20(21(32)13-17)27-23(39)8-7-22(35-27)18-3-2-12-34-14-18/h2-9,12-14,39H,15-16H2,1H3,(H2,36,37,41,42)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0770 | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102694
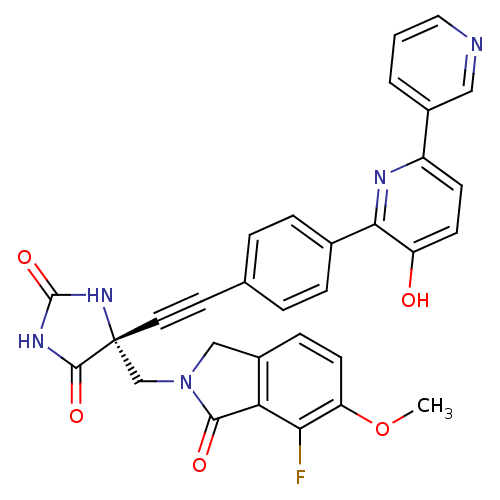
(US8541572, 1001)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3nc(ccc3O)-c3cccnc3)C(=O)c2c1F |r| Show InChI InChI=1S/C31H22FN5O5/c1-42-24-11-8-21-16-37(28(39)25(21)26(24)32)17-31(29(40)35-30(41)36-31)13-12-18-4-6-19(7-5-18)27-23(38)10-9-22(34-27)20-3-2-14-33-15-20/h2-11,14-15,38H,16-17H2,1H3,(H2,35,36,40,41)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0781 | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102696
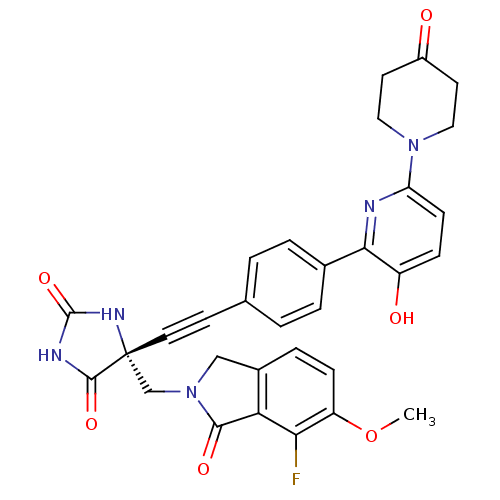
(US8541572, 1003)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3nc(ccc3O)N3CCC(=O)CC3)C(=O)c2c1F |r| Show InChI InChI=1S/C31H26FN5O6/c1-43-23-8-6-20-16-37(28(40)25(20)26(23)32)17-31(29(41)34-30(42)35-31)13-10-18-2-4-19(5-3-18)27-22(39)7-9-24(33-27)36-14-11-21(38)12-15-36/h2-9,39H,11-12,14-17H2,1H3,(H2,34,35,41,42)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0826 | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102855

(US8541572, 943)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(N=O)N3CCN(C)CC3)C(=O)c2c1 |r| Show InChI InChI=1S/C27H28N6O5/c1-31-11-13-32(14-12-31)23(30-37)19-5-3-18(4-6-19)9-10-27(25(35)28-26(36)29-27)17-33-16-20-7-8-21(38-2)15-22(20)24(33)34/h3-8,15,23H,11-14,16-17H2,1-2H3,(H2,28,29,35,36)/t23?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0830 | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50484748
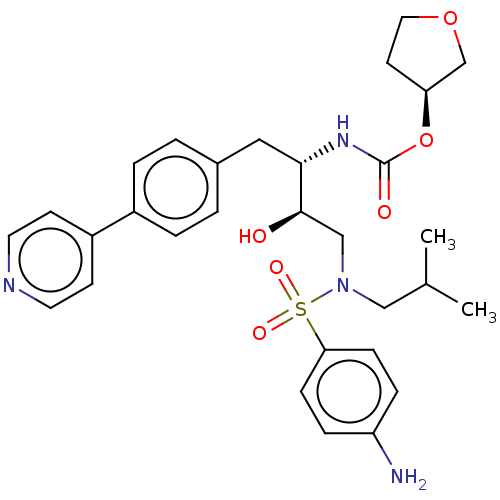
(CHEMBL1957077)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccc(cc1)-c1ccncc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C30H38N4O6S/c1-21(2)18-34(41(37,38)27-9-7-25(31)8-10-27)19-29(35)28(33-30(36)40-26-13-16-39-20-26)17-22-3-5-23(6-4-22)24-11-14-32-15-12-24/h3-12,14-15,21,26,28-29,35H,13,16-20,31H2,1-2H3,(H,33,36)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis |
Bioorg Med Chem Lett 22: 1976-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.037
BindingDB Entry DOI: 10.7270/Q2V69ND3 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM370121
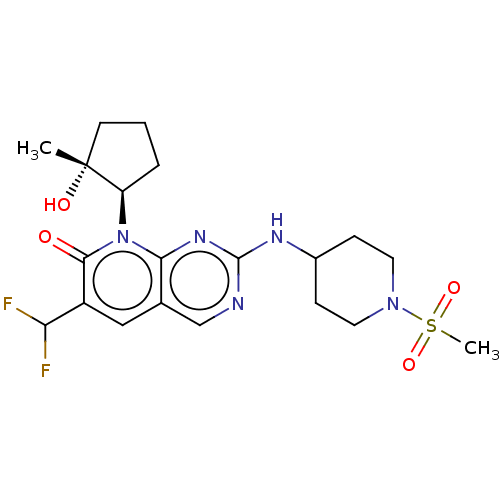
(6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...)Show SMILES C[C@@]1(O)CCC[C@H]1n1c2nc(NC3CCN(CC3)S(C)(=O)=O)ncc2cc(C(F)F)c1=O |r| Show InChI InChI=1S/C20H27F2N5O4S/c1-20(29)7-3-4-15(20)27-17-12(10-14(16(21)22)18(27)28)11-23-19(25-17)24-13-5-8-26(9-6-13)32(2,30)31/h10-11,13,15-16,29H,3-9H2,1-2H3,(H,23,24,25)/t15-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102852
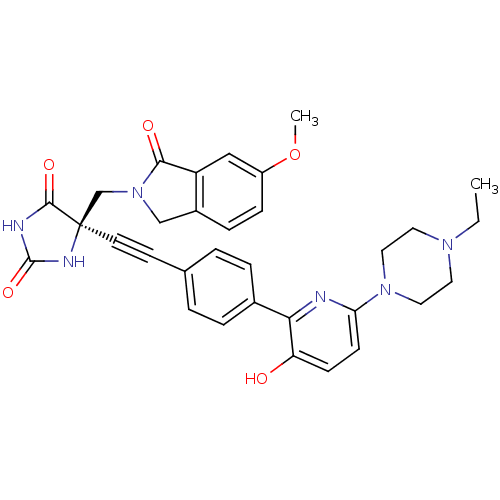
(US8541572, 936)Show SMILES CCN1CCN(CC1)c1ccc(O)c(n1)-c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H32N6O5/c1-3-36-14-16-37(17-15-36)27-11-10-26(39)28(33-27)22-6-4-21(5-7-22)12-13-32(30(41)34-31(42)35-32)20-38-19-23-8-9-24(43-2)18-25(23)29(38)40/h4-11,18,39H,3,14-17,19-20H2,1-2H3,(H2,34,35,41,42)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0930 | n/a | 72.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102843
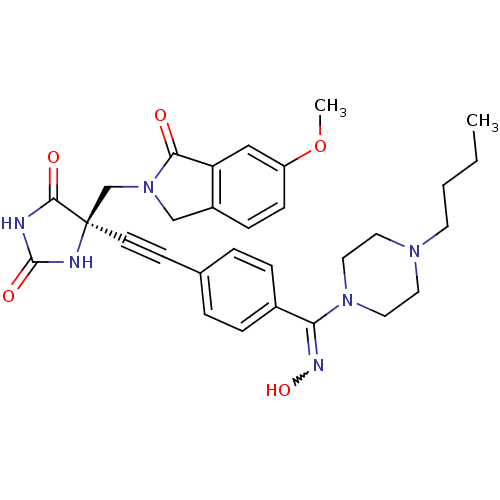
(US8541572, 925)Show SMILES CCCCN1CCN(CC1)C(=NO)c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:11.12| Show InChI InChI=1S/C30H34N6O5/c1-3-4-13-34-14-16-35(17-15-34)26(33-40)22-7-5-21(6-8-22)11-12-30(28(38)31-29(39)32-30)20-36-19-23-9-10-24(41-2)18-25(23)27(36)37/h5-10,18,40H,3-4,13-17,19-20H2,1-2H3,(H2,31,32,38,39)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0930 | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102679
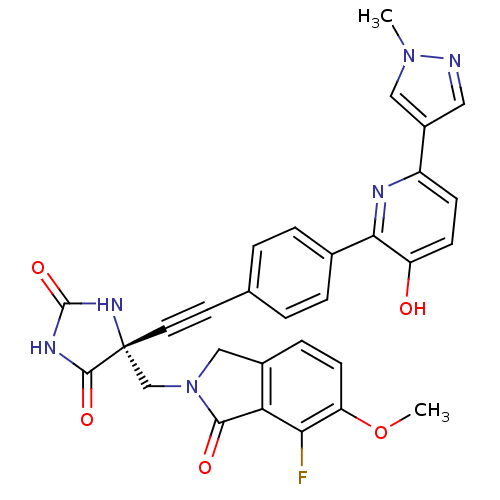
(US8541572, 986)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3nc(ccc3O)-c3cnn(C)c3)C(=O)c2c1F |r| Show InChI InChI=1S/C30H23FN6O5/c1-36-14-20(13-32-36)21-8-9-22(38)26(33-21)18-5-3-17(4-6-18)11-12-30(28(40)34-29(41)35-30)16-37-15-19-7-10-23(42-2)25(31)24(19)27(37)39/h3-10,13-14,38H,15-16H2,1-2H3,(H2,34,35,40,41)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0973 | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102669
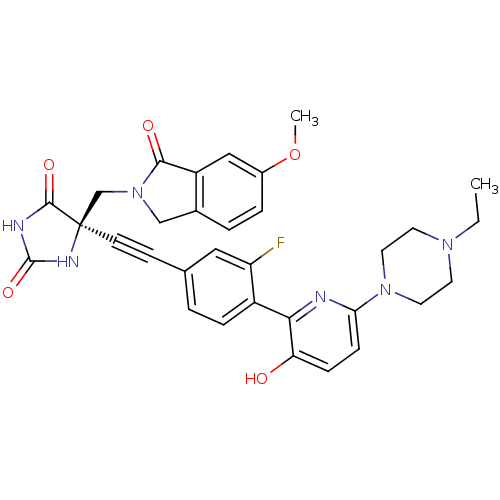
(CHEMBL1288726 | US8541572, 976)Show SMILES CCN1CCN(CC1)c1ccc(O)c(n1)-c1ccc(cc1F)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H31FN6O5/c1-3-37-12-14-38(15-13-37)27-9-8-26(40)28(34-27)23-7-4-20(16-25(23)33)10-11-32(30(42)35-31(43)36-32)19-39-18-21-5-6-22(44-2)17-24(21)29(39)41/h4-9,16-17,40H,3,12-15,18-19H2,1-2H3,(H2,35,36,42,43)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0988 | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102863
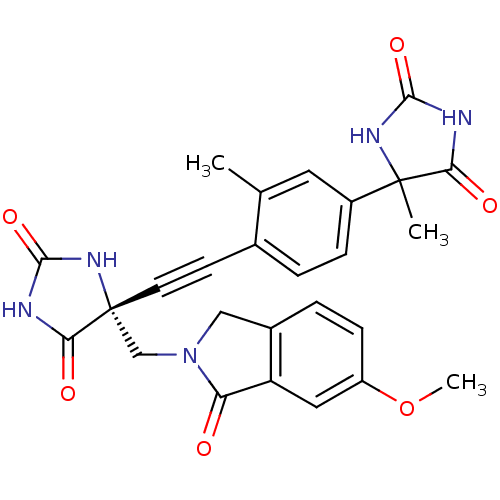
(US8541572, 2257)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3C)C3(C)NC(=O)NC3=O)C(=O)c2c1 |r| Show InChI InChI=1S/C26H23N5O6/c1-14-10-17(25(2)21(33)27-23(35)29-25)6-4-15(14)8-9-26(22(34)28-24(36)30-26)13-31-12-16-5-7-18(37-3)11-19(16)20(31)32/h4-7,10-11H,12-13H2,1-3H3,(H2,27,29,33,35)(H2,28,30,34,36)/t25?,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM102669

(CHEMBL1288726 | US8541572, 976)Show SMILES CCN1CCN(CC1)c1ccc(O)c(n1)-c1ccc(cc1F)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H31FN6O5/c1-3-37-12-14-38(15-13-37)27-9-8-26(40)28(34-27)23-7-4-20(16-25(23)33)10-11-32(30(42)35-31(43)36-32)19-39-18-21-5-6-22(44-2)17-24(21)29(39)41/h4-9,16-17,40H,3,12-15,18-19H2,1-2H3,(H2,35,36,42,43)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50092959
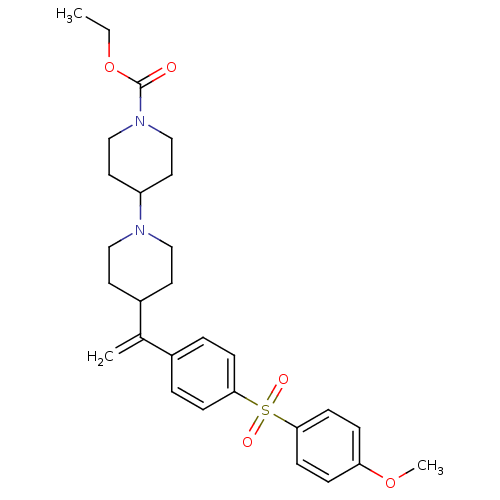
(4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...)Show SMILES CCOC(=O)N1CCC(CC1)N1CCC(CC1)C(=C)c1ccc(cc1)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C28H36N2O5S/c1-4-35-28(31)30-19-15-24(16-20-30)29-17-13-23(14-18-29)21(2)22-5-9-26(10-6-22)36(32,33)27-11-7-25(34-3)8-12-27/h5-12,23-24H,2,4,13-20H2,1,3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
Bioorg Med Chem Lett 17: 2260-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.058
BindingDB Entry DOI: 10.7270/Q2668H0S |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102844
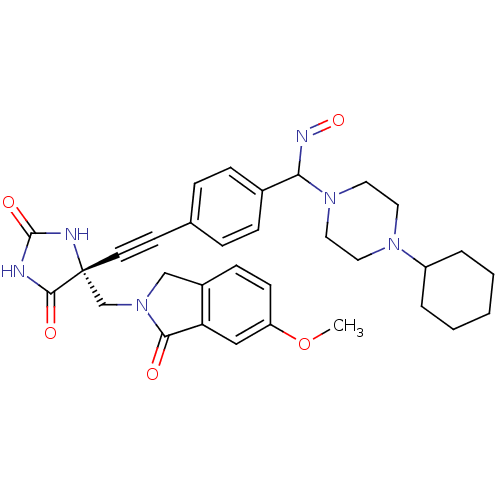
(US8541572, 926)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(N=O)N3CCN(CC3)C3CCCCC3)C(=O)c2c1 |r| Show InChI InChI=1S/C32H36N6O5/c1-43-26-12-11-24-20-38(29(39)27(24)19-26)21-32(30(40)33-31(41)34-32)14-13-22-7-9-23(10-8-22)28(35-42)37-17-15-36(16-18-37)25-5-3-2-4-6-25/h7-12,19,25,28H,2-6,15-18,20-21H2,1H3,(H2,33,34,40,41)/t28?,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102873

(US8541572, 2267)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccnc3)C(=O)c2c1F |r| Show InChI InChI=1S/C20H15FN4O4/c1-29-14-5-4-13-10-25(17(26)15(13)16(14)21)11-20(18(27)23-19(28)24-20)7-6-12-3-2-8-22-9-12/h2-5,8-9H,10-11H2,1H3,(H2,23,24,27,28)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102747
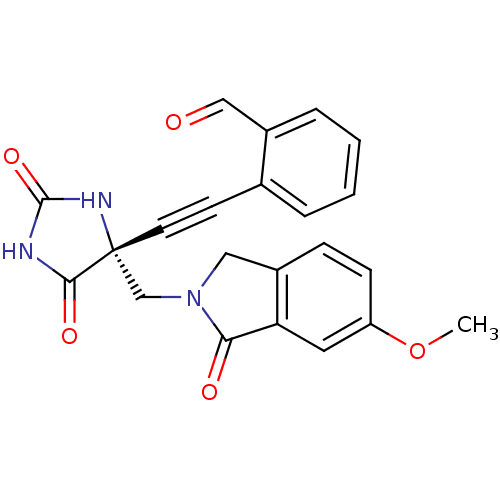
(US8541572, 1918)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3C=O)C(=O)c2c1 |r| Show InChI InChI=1S/C22H17N3O5/c1-30-17-7-6-15-11-25(19(27)18(15)10-17)13-22(20(28)23-21(29)24-22)9-8-14-4-2-3-5-16(14)12-26/h2-7,10,12H,11,13H2,1H3,(H2,23,24,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | n/a | 936 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102681
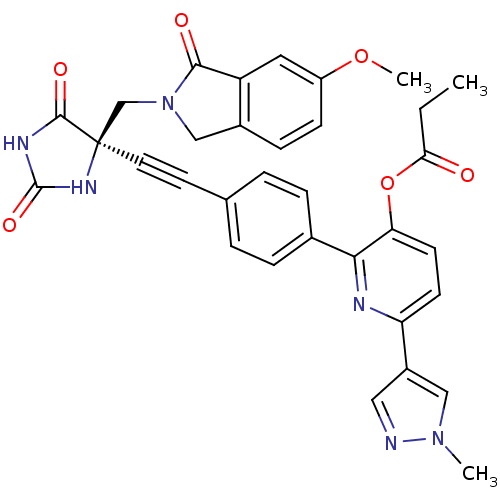
(US8541572, 988)Show SMILES CCC(=O)Oc1ccc(nc1-c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O)-c1cnn(C)c1 |r| Show InChI InChI=1S/C33H28N6O6/c1-4-28(40)45-27-12-11-26(23-16-34-38(2)17-23)35-29(27)21-7-5-20(6-8-21)13-14-33(31(42)36-32(43)37-33)19-39-18-22-9-10-24(44-3)15-25(22)30(39)41/h5-12,15-17H,4,18-19H2,1-3H3,(H2,36,37,42,43)/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.115 | n/a | 55.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102731
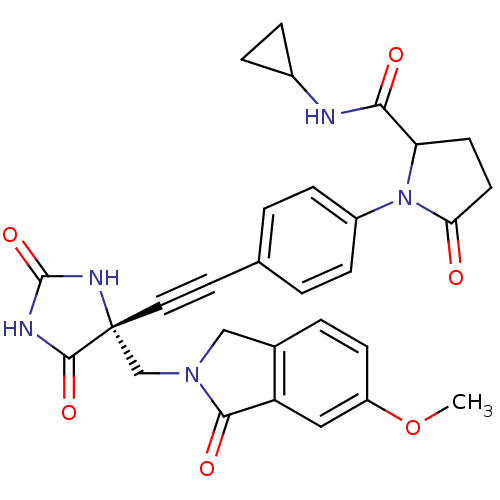
(US8541572, 1902)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)N3C(CCC3=O)C(=O)NC3CC3)C(=O)c2c1 |r| Show InChI InChI=1S/C29H27N5O6/c1-40-21-9-4-18-15-33(26(37)22(18)14-21)16-29(27(38)31-28(39)32-29)13-12-17-2-7-20(8-3-17)34-23(10-11-24(34)35)25(36)30-19-5-6-19/h2-4,7-9,14,19,23H,5-6,10-11,15-16H2,1H3,(H,30,36)(H2,31,32,38,39)/t23?,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.120 | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102857
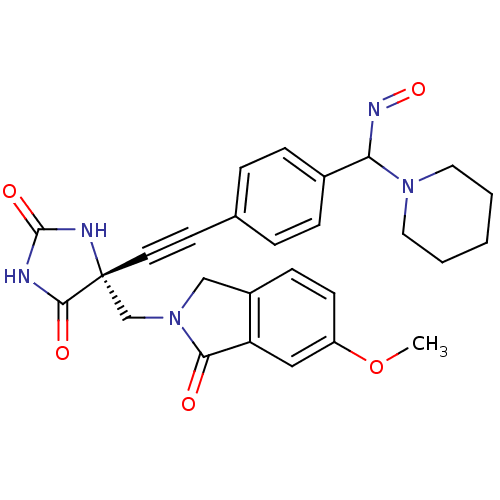
(US8541572, 946)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(N=O)N3CCCCC3)C(=O)c2c1 |r| Show InChI InChI=1S/C27H27N5O5/c1-37-21-10-9-20-16-32(24(33)22(20)15-21)17-27(25(34)28-26(35)29-27)12-11-18-5-7-19(8-6-18)23(30-36)31-13-3-2-4-14-31/h5-10,15,23H,2-4,13-14,16-17H2,1H3,(H2,28,29,34,35)/t23?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.120 | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102858
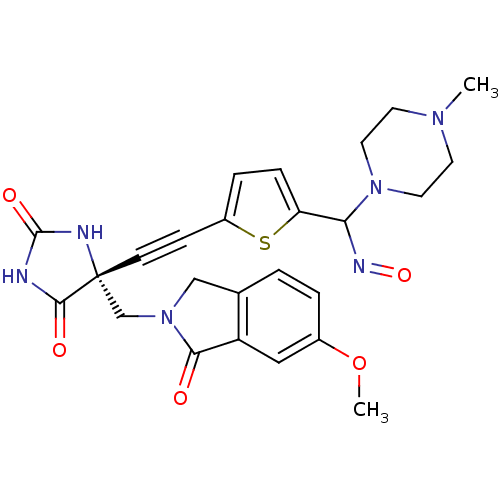
(US8541572, 951)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(s3)C(N=O)N3CCN(C)CC3)C(=O)c2c1 |r| Show InChI InChI=1S/C25H26N6O5S/c1-29-9-11-30(12-10-29)21(28-35)20-6-5-18(37-20)7-8-25(23(33)26-24(34)27-25)15-31-14-16-3-4-17(36-2)13-19(16)22(31)32/h3-6,13,21H,9-12,14-15H2,1-2H3,(H2,26,27,33,34)/t21?,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.120 | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102872
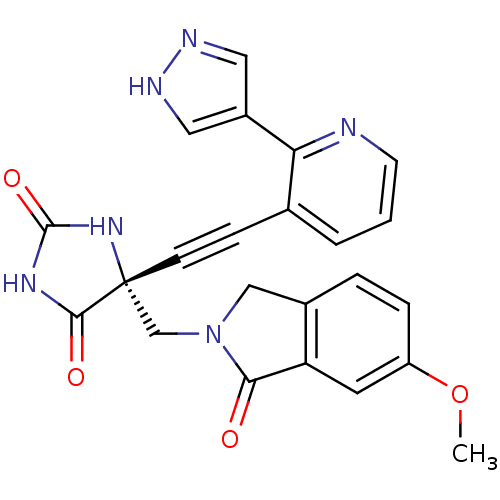
(US8541572, 2266)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccnc3-c3cn[nH]c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N6O4/c1-33-17-5-4-15-12-29(20(30)18(15)9-17)13-23(21(31)27-22(32)28-23)7-6-14-3-2-8-24-19(14)16-10-25-26-11-16/h2-5,8-11H,12-13H2,1H3,(H,25,26)(H2,27,28,31,32)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.120 | n/a | 927 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102853
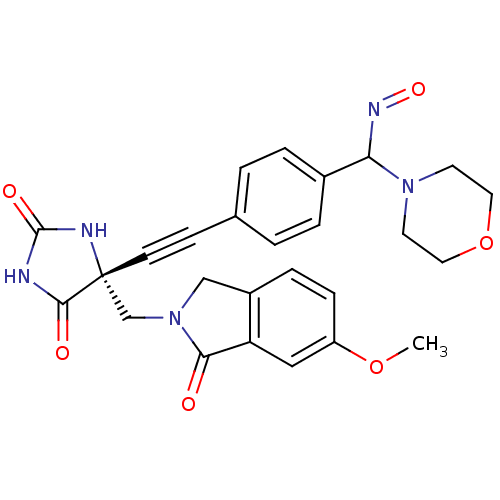
(US8541572, 941)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(N=O)N3CCOCC3)C(=O)c2c1 |r| Show InChI InChI=1S/C26H25N5O6/c1-36-20-7-6-19-15-31(23(32)21(19)14-20)16-26(24(33)27-25(34)28-26)9-8-17-2-4-18(5-3-17)22(29-35)30-10-12-37-13-11-30/h2-7,14,22H,10-13,15-16H2,1H3,(H2,27,28,33,34)/t22?,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102730
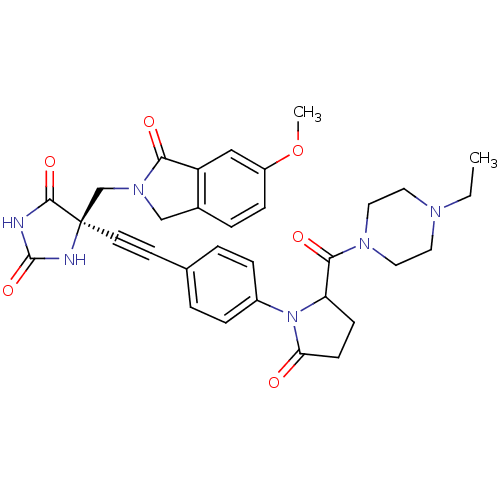
(US8541572, 1901)Show SMILES CCN1CCN(CC1)C(=O)C1CCC(=O)N1c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H34N6O6/c1-3-35-14-16-36(17-15-35)29(41)26-10-11-27(39)38(26)23-7-4-21(5-8-23)12-13-32(30(42)33-31(43)34-32)20-37-19-22-6-9-24(44-2)18-25(22)28(37)40/h4-9,18,26H,3,10-11,14-17,19-20H2,1-2H3,(H2,33,34,42,43)/t26?,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.130 | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM370121

(6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...)Show SMILES C[C@@]1(O)CCC[C@H]1n1c2nc(NC3CCN(CC3)S(C)(=O)=O)ncc2cc(C(F)F)c1=O |r| Show InChI InChI=1S/C20H27F2N5O4S/c1-20(29)7-3-4-15(20)27-17-12(10-14(16(21)22)18(27)28)11-23-19(25-17)24-13-5-8-26(9-6-13)32(2,30)31/h10-11,13,15-16,29H,3-9H2,1-2H3,(H,23,24,25)/t15-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against CCK2 receptor estimated from single shifts of pentagastrin concentration-effect curves in the isolated rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332265

((R)-5-((2-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3F)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-15-7-6-14-11-25(18(26)16(14)10-15)12-21(19(27)23-20(28)24-21)9-8-13-4-2-3-5-17(13)22/h2-7,10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50367123
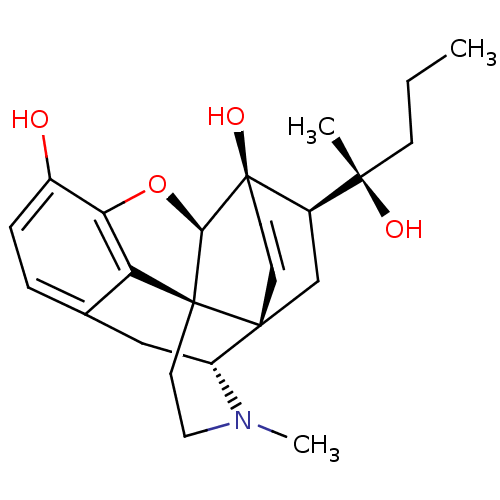
(ETORPHINE | M99)Show SMILES CCC[C@](C)(O)[C@H]1C[C@]23C=C[C@]1(O)[C@@H]1Oc4c5c(C[C@H]2N(C)CC[C@@]315)ccc4O |c:9,TLB:3:6:9.10:24.13,THB:7:8:16.17.18:20.23.22,9:8:16.17.18:20.23.22| Show InChI InChI=1S/C24H31NO4/c1-4-7-21(2,27)16-13-22-8-9-24(16,28)20-23(22)10-11-25(3)17(22)12-14-5-6-15(26)19(29-20)18(14)23/h5-6,8-9,16-17,20,26-28H,4,7,10-13H2,1-3H3/t16-,17-,20-,21+,22-,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 1301-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.078
BindingDB Entry DOI: 10.7270/Q2MP546B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377656

(CHEMBL259534)Show SMILES Clc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H17ClN4O3/c25-17-10-13-21(26-15-17)28-24(32)19-5-1-2-6-20(19)27-23(31)16-8-11-18(12-9-16)29-14-4-3-7-22(29)30/h1-15H,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using acromogenic substrate S-2222 preincubated for 30 mins before substrate addition measured after 20 mins by spectr... |
Eur J Med Chem 95: 388-99 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.052
BindingDB Entry DOI: 10.7270/Q29G5PH4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50123599

(ETORPHINE)Show SMILES [H][C@@]1(C[C@]23C=C[C@]1(OC)[C@@H]1Oc4c5c(C[C@H]2N(C)CC[C@@]315)ccc4O)[C@](C)(O)CCC |r,wU:15.17,20.21,25.33,1.0,wD:9.10,3.3,6.7,c:4,THB:17:16:13.12.14:3,(12.5,-39.62,;11.02,-39.23,;12.37,-38.48,;12.38,-36.94,;11.6,-38.27,;10.46,-37.1,;9.7,-38.44,;8.61,-39.53,;7.12,-39.12,;9.72,-36.9,;8.94,-35.56,;9.73,-33.87,;11.06,-34.63,;12.4,-33.87,;13.73,-34.64,;13.72,-36.18,;14.48,-34.84,;15.81,-34.06,;12.94,-34.84,;12.54,-35.75,;11.06,-36.16,;12.39,-32.32,;11.06,-31.55,;9.73,-32.32,;8.39,-31.55,;11,-40.77,;10.98,-42.3,;9.46,-40.75,;12.54,-40.79,;13.29,-42.14,;14.83,-42.16,)| Show InChI InChI=1S/C25H33NO4/c1-5-8-22(2,28)17-14-23-9-10-25(17,29-4)21-24(23)11-12-26(3)18(23)13-15-6-7-16(27)20(30-21)19(15)24/h6-7,9-10,17-18,21,27-28H,5,8,11-14H2,1-4H3/t17-,18-,21-,22-,23-,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in CHO cell membranes |
Bioorg Med Chem Lett 25: 4689-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.092
BindingDB Entry DOI: 10.7270/Q2NV9M2W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332262
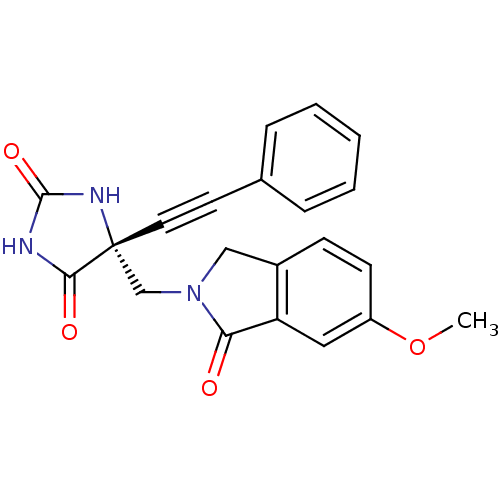
((R)-5-((6-methoxy-1-oxoisoindolin-2-yl)methyl)-5-(...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H17N3O4/c1-28-16-8-7-15-12-24(18(25)17(15)11-16)13-21(19(26)22-20(27)23-21)10-9-14-5-3-2-4-6-14/h2-8,11H,12-13H2,1H3,(H2,22,23,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM102846
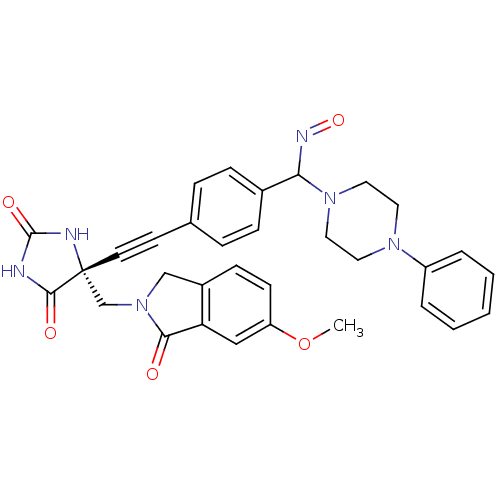
(US8541572, 928)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(N=O)N3CCN(CC3)c3ccccc3)C(=O)c2c1 |r| Show InChI InChI=1S/C32H30N6O5/c1-43-26-12-11-24-20-38(29(39)27(24)19-26)21-32(30(40)33-31(41)34-32)14-13-22-7-9-23(10-8-22)28(35-42)37-17-15-36(16-18-37)25-5-3-2-4-6-25/h2-12,19,28H,15-18,20-21H2,1H3,(H2,33,34,40,41)/t28?,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.150 | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Inhibition assay using TNF-alpha. |
US Patent US8541572 (2013)
BindingDB Entry DOI: 10.7270/Q2QC024J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data