| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50210170 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1635124 (CHEMBL3878022) |
|---|
| Ki | 3430±n/a nM |
|---|
| Citation |  Loesche, A; Wiese, J; Sommerwerk, S; Simon, V; Brandt, W; Csuk, R Repurposing N,N'-bis-(arylamidino)-1,4-piperazinedicarboxamidines: An unexpected class of potent inhibitors of cholinesterases. Eur J Med Chem125:430-434 (2017) [PubMed] Article Loesche, A; Wiese, J; Sommerwerk, S; Simon, V; Brandt, W; Csuk, R Repurposing N,N'-bis-(arylamidino)-1,4-piperazinedicarboxamidines: An unexpected class of potent inhibitors of cholinesterases. Eur J Med Chem125:430-434 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_ELEEL | Acetylcholinesterase (AChE) | Acetylcholinesterase (EeAChE) | ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 71812.79 |
|---|
| Organism: | Electrophorus electricus (Electric eel) |
|---|
| Description: | n/a |
|---|
| Residue: | 633 |
|---|
| Sequence: | MKILDALLFPVIFIMFFIHLSIAQTDPELTIMTRLGQVQGTRLPVPDRSHVIAFLGIPFA
EPPLGKMRFKPPEPKKPWNDVFDARDYPSACYQYVDTSYPGFSGTEMWNPNRMMSEDCLY
LNVWVPATPRPHNLTVMVWIYGGGFYSGSSSLDVYDGRYLAHSEKVVVVSMNYRVSAFGF
LALNGSAEAPGNVGLLDQRLALQWVQDNIHFFGGNPKQVTIFGESAGAASVGMHLLSPDS
RPKFTRAILQSGVPNGPWRTVSFDEARRRAIKLGRLVGCPDGNDTDLIDCLRSKQPQDLI
DQEWLVLPFSGLFRFSFVPVIDGVVFPDTPEAMLNSGNFKDTQILLGVNQNEGSYFLIYG
APGFSKDNESLITREDFLQGVKMSVPHANEIGLEAVILQYTDWMDEDNPIKNREAMDDIV
GDHNVVCPLQHFAKMYAQYSILQGQTGTASQGNLGWGNSGSASNSGNSQVSVYLYMFDHR
ASNLVWPEWMGVIHGYEIEFVFGLPLEKRLNYTLEEEKLSRRMMKYWANFARTGNPNINV
DGSIDSRRRWPVFTSTEQKHVGLNTDSLKVHKGLKSQFCALWNRFLPRLLNVTENIDDAE
RQWKAEFHRWSSYMMHWKNQFDHYSKQERCTNL
|
|
|
|---|
| BDBM50210170 |
|---|
| n/a |
|---|
| Name | BDBM50210170 |
|---|
| Synonyms: | CHEMBL3894580 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H24Br2N10 |
|---|
| Mol. Mass. | 564.28 |
|---|
| SMILES | Brc1ccc(NC(=N)NC(=N)N2CCN(CC2)C(=N)NC(=N)Nc2ccc(Br)cc2)cc1 |
|---|
| Structure | 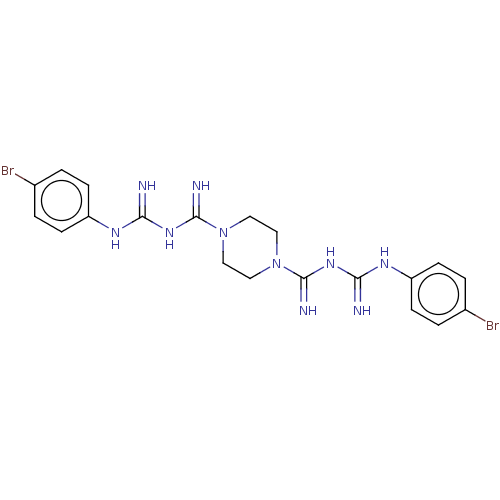
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Loesche, A; Wiese, J; Sommerwerk, S; Simon, V; Brandt, W; Csuk, R Repurposing N,N'-bis-(arylamidino)-1,4-piperazinedicarboxamidines: An unexpected class of potent inhibitors of cholinesterases. Eur J Med Chem125:430-434 (2017) [PubMed] Article
Loesche, A; Wiese, J; Sommerwerk, S; Simon, V; Brandt, W; Csuk, R Repurposing N,N'-bis-(arylamidino)-1,4-piperazinedicarboxamidines: An unexpected class of potent inhibitors of cholinesterases. Eur J Med Chem125:430-434 (2017) [PubMed] Article