| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Genome polyprotein |
|---|
| Ligand | BDBM61949 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | EMSA |
|---|
| pH | 7.5±n/a |
|---|
| Temperature | 296.15±n/a K |
|---|
| IC50 | 1.2e+4± 6e+2 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Mukherjee, S; Weiner, WS; Schroeder, CE; Simpson, DS; Hanson, AM; Sweeney, NL; Marvin, RK; Ndjomou, J; Kolli, R; Isailovic, D; Schoenen, FJ; Frick, DN Ebselen Inhibits Hepatitis C Virus NS3 Helicase Binding to Nucleic Acid and Prevents Viral Replication ACS Chem Biol9:2393-2403 (2014) [PubMed] Article Mukherjee, S; Weiner, WS; Schroeder, CE; Simpson, DS; Hanson, AM; Sweeney, NL; Marvin, RK; Ndjomou, J; Kolli, R; Isailovic, D; Schoenen, FJ; Frick, DN Ebselen Inhibits Hepatitis C Virus NS3 Helicase Binding to Nucleic Acid and Prevents Viral Replication ACS Chem Biol9:2393-2403 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Genome polyprotein |
|---|
| Name: | Genome polyprotein |
|---|
| Synonyms: | NS3 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 67067.41 |
|---|
| Organism: | Hepatitis C virus |
|---|
| Description: | gi_125541954 |
|---|
| Residue: | 631 |
|---|
| Sequence: | APITAYAQQTRGLLGCIITGLTGRDKNQVEGEVQIVSTAAQTFLATCINGVCWTVYHGAG
TRTIASSKGPVIQMYTNVDQDLVGWPAPQGARSLTPCTCGSSDLYLVTRHADVIPVRRRG
DGRGSLLSPRPISYLKGSSGGPLLCPAGHAVGIFRAAVCTRGVAKAVDFIPVEGLETTMR
SPVFSDNSSPPAVPQSYQVAHLHAPTGSGKSTKVPAAYAAQGYKVLVLNPSVAATLGFGA
YMSKAHGIDPNIRTGVRTITTGSPITYSTYGKFLADGGCSGSAYDIIICDECHSTDATSI
LGIGTVLDQAETAGARLTVLATATPPGSVTVPHPNIEEVALSTTGEIPFYGKAIPLEAIK
GGRHLIFCHSKKKCDELAAKLVALGVNAVAYYRGLDVSVIPASGDVVVVATDALMTGFTG
DFDSVIDCNTCVTQTVDFSLDPTFTIETTTLPQDAVSRTQRRGRTGRGKPGIYRFVTPGE
RPSGMFDSSVLCECYDAGCAWYELTPAETTVRLRAYMNTPGLPVCQDHLEFWEGVFTGLT
HIDAHFLSQTKQSGENLPYLVAYQATVCARAQAPPPSWDQMWKCLIRLKPTLHGPTPLLY
RLGAVQNEITLTHPITKYIMTCMSADLEVVT
|
|
|
|---|
| BDBM61949 |
|---|
| n/a |
|---|
| Name | BDBM61949 |
|---|
| Synonyms: | KSC-4-295 | KUC105342N | N-(3-bromophenyl)-2-(3-keto-1,2-benzothiazol-2-yl)acetamide | N-(3-bromophenyl)-2-(3-keto-1,2-benzothiazol-2-yl)acetamide (11) | N-(3-bromophenyl)-2-(3-oxidanylidene-1,2-benzothiazol-2-yl)ethanamide | N-(3-bromophenyl)-2-(3-oxo-1,2-benzothiazol-2-yl)acetamide | cid_45105114 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H11BrN2O2S |
|---|
| Mol. Mass. | 363.229 |
|---|
| SMILES | Brc1cccc(NC(=O)Cn2sc3ccccc3c2=O)c1 |
|---|
| Structure | 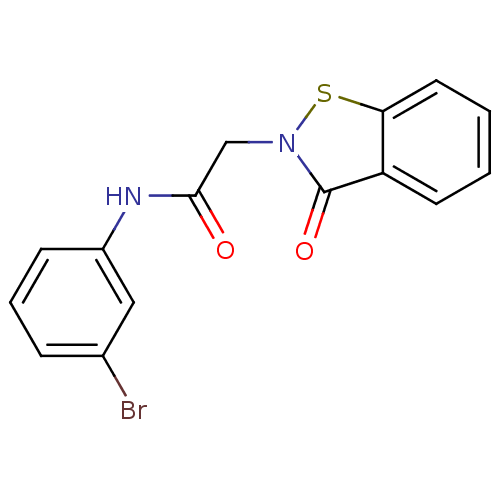
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Mukherjee, S; Weiner, WS; Schroeder, CE; Simpson, DS; Hanson, AM; Sweeney, NL; Marvin, RK; Ndjomou, J; Kolli, R; Isailovic, D; Schoenen, FJ; Frick, DN Ebselen Inhibits Hepatitis C Virus NS3 Helicase Binding to Nucleic Acid and Prevents Viral Replication ACS Chem Biol9:2393-2403 (2014) [PubMed] Article
Mukherjee, S; Weiner, WS; Schroeder, CE; Simpson, DS; Hanson, AM; Sweeney, NL; Marvin, RK; Ndjomou, J; Kolli, R; Isailovic, D; Schoenen, FJ; Frick, DN Ebselen Inhibits Hepatitis C Virus NS3 Helicase Binding to Nucleic Acid and Prevents Viral Replication ACS Chem Biol9:2393-2403 (2014) [PubMed] Article