| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone-lysine N-methyltransferase 2A |
|---|
| Ligand | BDBM553008 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Surface Plasmon Resonance (SPR) Assay |
|---|
| Kd | 3.5±n/a nM |
|---|
| Citation |  Al-Awar, R; Zepeda-Velazquez, CA; Poda, G; Isaac, M; Uehling, D; Wilson, B; Joseph, B; Liu, Y; Subramanian, P; Mamai, A; Prakesch, M; Stille, JK Substituted carboxamides as inhibitors of WDR5 protein-protein binding US Patent US11319299 Publication Date 5/3/2022 Al-Awar, R; Zepeda-Velazquez, CA; Poda, G; Isaac, M; Uehling, D; Wilson, B; Joseph, B; Liu, Y; Subramanian, P; Mamai, A; Prakesch, M; Stille, JK Substituted carboxamides as inhibitors of WDR5 protein-protein binding US Patent US11319299 Publication Date 5/3/2022 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone-lysine N-methyltransferase 2A |
|---|
| Name: | Histone-lysine N-methyltransferase 2A |
|---|
| Synonyms: | ALL-1 | ALL1 | ALL1 | C-terminal cleavage product of 180 kDa | CXXC-type zinc finger protein 7 | CXXC7 | HRX | HTRX | Histone-lysine N-methyltransferase 2A | KMT2A | KMT2A_HUMAN | Lysine N-methyltransferase 2A | MLL | MLL cleavage product C180 | MLL cleavage product N320 | MLL1 | Myeloid/lymphoid or mixed-lineage leukemia | Myeloid/lymphoid or mixed-lineage leukemia (MLL) | Myeloid/lymphoid or mixed-lineage leukemia protein 1 | N-terminal cleavage product of 320 kDa | TRX1 | Trithorax-like protein | Zinc finger protein HRX | p180 | p320 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 431905.67 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q03164 |
|---|
| Residue: | 3969 |
|---|
| Sequence: | MAHSCRWRFPARPGTTGGGGGGGRRGLGGAPRQRVPALLLPPGPPVGGGGPGAPPSPPAV
AAAAAAAGSSGAGVPGGAAAASAASSSSASSSSSSSSSASSGPALLRVGPGFDAALQVSA
AIGTNLRRFRAVFGESGGGGGSGEDEQFLGFGSDEEVRVRSPTRSPSVKTSPRKPRGRPR
SGSDRNSAILSDPSVFSPLNKSETKSGDKIKKKDSKSIEKKRGRPPTFPGVKIKITHGKD
ISELPKGNKEDSLKKIKRTPSATFQQATKIKKLRAGKLSPLKSKFKTGKLQIGRKGVQIV
RRRGRPPSTERIKTPSGLLINSELEKPQKVRKDKEGTPPLTKEDKTVVRQSPRRIKPVRI
IPSSKRTDATIAKQLLQRAKKGAQKKIEKEAAQLQGRKVKTQVKNIRQFIMPVVSAISSR
IIKTPRRFIEDEDYDPPIKIARLESTPNSRFSAPSCGSSEKSSAASQHSSQMSSDSSRSS
SPSVDTSTDSQASEEIQVLPEERSDTPEVHPPLPISQSPENESNDRRSRRYSVSERSFGS
RTTKKLSTLQSAPQQQTSSSPPPPLLTPPPPLQPASSISDHTPWLMPPTIPLASPFLPAS
TAPMQGKRKSILREPTFRWTSLKHSRSEPQYFSSAKYAKEGLIRKPIFDNFRPPPLTPED
VGFASGFSASGTAASARLFSPLHSGTRFDMHKRSPLLRAPRFTPSEAHSRIFESVTLPSN
RTSAGTSSSGVSNRKRKRKVFSPIRSEPRSPSHSMRTRSGRLSSSELSPLTPPSSVSSSL
SISVSPLATSALNPTFTFPSHSLTQSGESAEKNQRPRKQTSAPAEPFSSSSPTPLFPWFT
PGSQTERGRNKDKAPEELSKDRDADKSVEKDKSRERDREREKENKRESRKEKRKKGSEIQ
SSSALYPVGRVSKEKVVGEDVATSSSAKKATGRKKSSSHDSGTDITSVTLGDTTAVKTKI
LIKKGRGNLEKTNLDLGPTAPSLEKEKTLCLSTPSSSTVKHSTSSIGSMLAQADKLPMTD
KRVASLLKKAKAQLCKIEKSKSLKQTDQPKAQGQESDSSETSVRGPRIKHVCRRAAVALG
RKRAVFPDDMPTLSALPWEEREKILSSMGNDDKSSIAGSEDAEPLAPPIKPIKPVTRNKA
PQEPPVKKGRRSRRCGQCPGCQVPEDCGVCTNCLDKPKFGGRNIKKQCCKMRKCQNLQWM
PSKAYLQKQAKAVKKKEKKSKTSEKKDSKESSVVKNVVDSSQKPTPSAREDPAPKKSSSE
PPPRKPVEEKSEEGNVSAPGPESKQATTPASRKSSKQVSQPALVIPPQPPTTGPPRKEVP
KTTPSEPKKKQPPPPESGPEQSKQKKVAPRPSIPVKQKPKEKEKPPPVNKQENAGTLNIL
STLSNGNSSKQKIPADGVHRIRVDFKEDCEAENVWEMGGLGILTSVPITPRVVCFLCASS
GHVEFVYCQVCCEPFHKFCLEENERPLEDQLENWCCRRCKFCHVCGRQHQATKQLLECNK
CRNSYHPECLGPNYPTKPTKKKKVWICTKCVRCKSCGSTTPGKGWDAQWSHDFSLCHDCA
KLFAKGNFCPLCDKCYDDDDYESKMMQCGKCDRWVHSKCENLSDEMYEILSNLPESVAYT
CVNCTERHPAEWRLALEKELQISLKQVLTALLNSRTTSHLLRYRQAAKPPDLNPETEESI
PSRSSPEGPDPPVLTEVSKQDDQQPLDLEGVKRKMDQGNYTSVLEFSDDIVKIIQAAINS
DGGQPEIKKANSMVKSFFIRQMERVFPWFSVKKSRFWEPNKVSSNSGMLPNAVLPPSLDH
NYAQWQEREENSHTEQPPLMKKIIPAPKPKGPGEPDSPTPLHPPTPPILSTDRSREDSPE
LNPPPGIEDNRQCALCLTYGDDSANDAGRLLYIGQNEWTHVNCALWSAEVFEDDDGSLKN
VHMAVIRGKQLRCEFCQKPGATVGCCLTSCTSNYHFMCSRAKNCVFLDDKKVYCQRHRDL
IKGEVVPENGFEVFRRVFVDFEGISLRRKFLNGLEPENIHMMIGSMTIDCLGILNDLSDC
EDKLFPIGYQCSRVYWSTTDARKRCVYTCKIVECRPPVVEPDINSTVEHDENRTIAHSPT
SFTESSSKESQNTAEIISPPSPDRPPHSQTSGSCYYHVISKVPRIRTPSYSPTQRSPGCR
PLPSAGSPTPTTHEIVTVGDPLLSSGLRSIGSRRHSTSSLSPQRSKLRIMSPMRTGNTYS
RNNVSSVSTTGTATDLESSAKVVDHVLGPLNSSTSLGQNTSTSSNLQRTVVTVGNKNSHL
DGSSSSEMKQSSASDLVSKSSSLKGEKTKVLSSKSSEGSAHNVAYPGIPKLAPQVHNTTS
RELNVSKIGSFAEPSSVSFSSKEALSFPHLHLRGQRNDRDQHTDSTQSANSSPDEDTEVK
TLKLSGMSNRSSIINEHMGSSSRDRRQKGKKSCKETFKEKHSSKSFLEPGQVTTGEEGNL
KPEFMDEVLTPEYMGQRPCNNVSSDKIGDKGLSMPGVPKAPPMQVEGSAKELQAPRKRTV
KVTLTPLKMENESQSKNALKESSPASPLQIESTSPTEPISASENPGDGPVAQPSPNNTSC
QDSQSNNYQNLPVQDRNLMLPDGPKPQEDGSFKRRYPRRSARARSNMFFGLTPLYGVRSY
GEEDIPFYSSSTGKKRGKRSAEGQVDGADDLSTSDEDDLYYYNFTRTVISSGGEERLASH
NLFREEEQCDLPKISQLDGVDDGTESDTSVTATTRKSSQIPKRNGKENGTENLKIDRPED
AGEKEHVTKSSVGHKNEPKMDNCHSVSRVKTQGQDSLEAQLSSLESSRRVHTSTPSDKNL
LDTYNTELLKSDSDNNNSDDCGNILPSDIMDFVLKNTPSMQALGESPESSSSELLNLGEG
LGLDSNREKDMGLFEVFSQQLPTTEPVDSSVSSSISAEEQFELPLELPSDLSVLTTRSPT
VPSQNPSRLAVISDSGEKRVTITEKSVASSESDPALLSPGVDPTPEGHMTPDHFIQGHMD
ADHISSPPCGSVEQGHGNNQDLTRNSSTPGLQVPVSPTVPIQNQKYVPNSTDSPGPSQIS
NAAVQTTPPHLKPATEKLIVVNQNMQPLYVLQTLPNGVTQKIQLTSSVSSTPSVMETNTS
VLGPMGGGLTLTTGLNPSLPTSQSLFPSASKGLLPMSHHQHLHSFPAATQSSFPPNISNP
PSGLLIGVQPPPDPQLLVSESSQRTDLSTTVATPSSGLKKRPISRLQTRKNKKLAPSSTP
SNIAPSDVVSNMTLINFTPSQLPNHPSLLDLGSLNTSSHRTVPNIIKRSKSSIMYFEPAP
LLPQSVGGTAATAAGTSTISQDTSHLTSGSVSGLASSSSVLNVVSMQTTTTPTSSASVPG
HVTLTNPRLLGTPDIGSISNLLIKASQQSLGIQDQPVALPPSSGMFPQLGTSQTPSTAAI
TAASSICVLPSTQTTGITAASPSGEADEHYQLQHVNQLLASKTGIHSSQRDLDSASGPQV
SNFTQTVDAPNSMGLEQNKALSSAVQASPTSPGGSPSSPSSGQRSASPSVPGPTKPKPKT
KRFQLPLDKGNGKKHKVSHLRTSSSEAHIPDQETTSLTSGTGTPGAEAEQQDTASVEQSS
QKECGQPAGQVAVLPEVQVTQNPANEQESAEPKTVEEEESNFSSPLMLWLQQEQKRKESI
TEKKPKKGLVFEISSDDGFQICAESIEDAWKSLTDKVQEARSNARLKQLSFAGVNGLRML
GILHDAVVFLIEQLSGAKHCRNYKFRFHKPEEANEPPLNPHGSARAEVHLRKSAFDMFNF
LASKHRQPPEYNPNDEEEEEVQLKSARRATSMDLPMPMRFRHLKKTSKEAVGVYRSPIHG
RGLFCKRNIDAGEMVIEYAGNVIRSIQTDKREKYYDSKGIGCYMFRIDDSEVVDATMHGN
AARFINHSCEPNCYSRVINIDGQKHIVIFAMRKIYRGEELTYDYKFPIEDASNKLPCNCG
AKKCRKFLN
|
|
|
|---|
| BDBM553008 |
|---|
| n/a |
|---|
| Name | BDBM553008 |
|---|
| Synonyms: | N-(5-(2- morpholinopyrimidin-5- yl)-2-((3R,5S)-3,4,5- trimethylpiperazin-1- yl)phenyl)-6-oxo-4- (trifluoromethyl)-1,6- dihydropyridine-3- carboxamide | US11319299, Example Table6.58 | US11319299, Comp. No. 58 | US11319299, Example 58 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H32F3N7O3 |
|---|
| Mol. Mass. | 571.594 |
|---|
| SMILES | C[C@H]1CN(C[C@@H](C)N1C)c1ccc(cc1NC(=O)c1c[nH]c(=O)cc1C(F)(F)F)-c1cnc(nc1)N1CCOCC1 |r| |
|---|
| Structure | 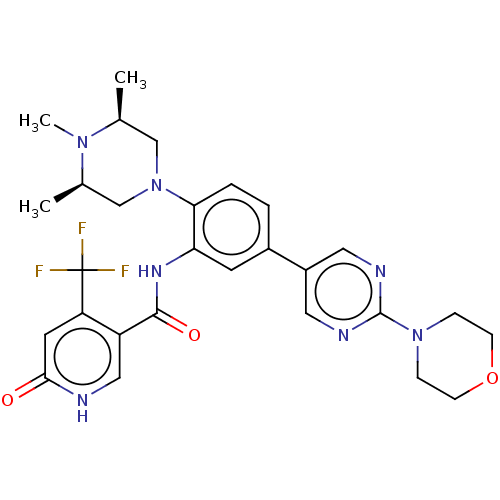
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Al-Awar, R; Zepeda-Velazquez, CA; Poda, G; Isaac, M; Uehling, D; Wilson, B; Joseph, B; Liu, Y; Subramanian, P; Mamai, A; Prakesch, M; Stille, JK Substituted carboxamides as inhibitors of WDR5 protein-protein binding US Patent US11319299 Publication Date 5/3/2022
Al-Awar, R; Zepeda-Velazquez, CA; Poda, G; Isaac, M; Uehling, D; Wilson, B; Joseph, B; Liu, Y; Subramanian, P; Mamai, A; Prakesch, M; Stille, JK Substituted carboxamides as inhibitors of WDR5 protein-protein binding US Patent US11319299 Publication Date 5/3/2022