| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Dipeptidyl peptidase 8 |
|---|
| Ligand | BDBM11464 |
|---|
| Substrate/Competitor | BDBM11526 |
|---|
| Meas. Tech. | Enzyme Inhibition Assay |
|---|
| pH | 8±n/a |
|---|
| Temperature | 310.15±n/a K |
|---|
| IC50 | 364±n/a nM |
|---|
| Citation |  Jiaang, WT; Chen, YS; Hsu, T; Wu, SH; Chien, CH; Chang, CN; Chang, SP; Lee, SJ; Chen, X Novel isoindoline compounds for potent and selective inhibition of prolyl dipeptidase DPP8. Bioorg Med Chem Lett15:687-91 (2005) [PubMed] Article Jiaang, WT; Chen, YS; Hsu, T; Wu, SH; Chien, CH; Chang, CN; Chang, SP; Lee, SJ; Chen, X Novel isoindoline compounds for potent and selective inhibition of prolyl dipeptidase DPP8. Bioorg Med Chem Lett15:687-91 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method |
|---|
| |
| Dipeptidyl peptidase 8 |
|---|
| Name: | Dipeptidyl peptidase 8 |
|---|
| Synonyms: | DPP8 | DPP8_HUMAN | DPRP-1 | DPRP1 | Dipeptidyl peptidase 8 (DPP-8) | Dipeptidyl peptidase 8 (DPP8) | Dipeptidyl peptidase 8/9 | Dipeptidyl peptidase IV-related protein 1 | Dipeptidyl peptidase VIII | Dipeptidyl peptidase VIII (DDP-VIII) | Prolyl dipeptidase DPP8 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 103342.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q6V1X1 |
|---|
| Residue: | 898 |
|---|
| Sequence: | MWKRSEQMKIKSGKCNMAAAMETEQLGVEIFETADCEENIESQDRPKLEPFYVERYSWSQ
LKKLLADTRKYHGYMMAKAPHDFMFVKRNDPDGPHSDRIYYLAMSGENRENTLFYSEIPK
TINRAAVLMLSWKPLLDLFQATLDYGMYSREEELLRERKRIGTVGIASYDYHQGSGTFLF
QAGSGIYHVKDGGPQGFTQQPLRPNLVETSCPNIRMDPKLCPADPDWIAFIHSNDIWISN
IVTREERRLTYVHNELANMEEDARSAGVATFVLQEEFDRYSGYWWCPKAETTPSGGKILR
ILYEENDESEVEIIHVTSPMLETRRADSFRYPKTGTANPKVTFKMSEIMIDAEGRIIDVI
DKELIQPFEILFEGVEYIARAGWTPEGKYAWSILLDRSQTRLQIVLISPELFIPVEDDVM
ERQRLIESVPDSVTPLIIYEETTDIWINIHDIFHVFPQSHEEEIEFIFASECKTGFRHLY
KITSILKESKYKRSSGGLPAPSDFKCPIKEEIAITSGEWEVLGRHGSNIQVDEVRRLVYF
EGTKDSPLEHHLYVVSYVNPGEVTRLTDRGYSHSCCISQHCDFFISKYSNQKNPHCVSLY
KLSSPEDDPTCKTKEFWATILDSAGPLPDYTPPEIFSFESTTGFTLYGMLYKPHDLQPGK
KYPTVLFIYGGPQVQLVNNRFKGVKYFRLNTLASLGYVVVVIDNRGSCHRGLKFEGAFKY
KMGQIEIDDQVEGLQYLASRYDFIDLDRVGIHGWSYGGYLSLMALMQRSDIFRVAIAGAP
VTLWIFYDTGYTERYMGHPDQNEQGYYLGSVAMQAEKFPSEPNRLLLLHGFLDENVHFAH
TSILLSFLVRAGKPYDLQIYPQERHSIRVPESGEHYELHLLHYLQENLGSRIAALKVI
|
|
|
|---|
| BDBM11464 |
|---|
| BDBM11526 |
|---|
| Name | BDBM11464 |
|---|
| Synonyms: | (2S,3S)-2-amino-3-methyl-1-(1,3-thiazolidin-3-yl)pentan-1-one | BMCL15687 Compound 4 | CHEMBL98408 | P32-98 | thiazolidide 2 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C9H18N2OS |
|---|
| Mol. Mass. | 202.317 |
|---|
| SMILES | CC[C@H](C)[C@H](N)C(=O)N1CCSC1 |r| |
|---|
| Structure | 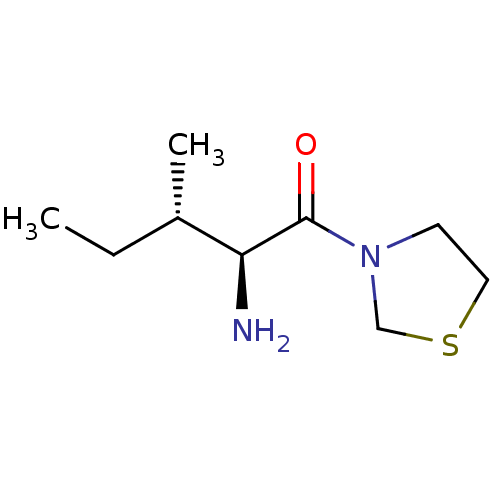
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jiaang, WT; Chen, YS; Hsu, T; Wu, SH; Chien, CH; Chang, CN; Chang, SP; Lee, SJ; Chen, X Novel isoindoline compounds for potent and selective inhibition of prolyl dipeptidase DPP8. Bioorg Med Chem Lett15:687-91 (2005) [PubMed] Article
Jiaang, WT; Chen, YS; Hsu, T; Wu, SH; Chien, CH; Chang, CN; Chang, SP; Lee, SJ; Chen, X Novel isoindoline compounds for potent and selective inhibition of prolyl dipeptidase DPP8. Bioorg Med Chem Lett15:687-91 (2005) [PubMed] Article