

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | 11-beta-hydroxysteroid dehydrogenase type 2 | ||
| Ligand | BDBM13756 | ||
| Substrate/Competitor | BDBM13775 | ||
| Meas. Tech. | Human and Mouse 11beta-HSD1 SPA Assay | ||
| IC50 | 23000±n/a nM | ||
| Citation |  Patel, JR; Shuai, Q; Dinges, J; Winn, M; Pliushchev, M; Fung, S; Monzon, K; Chiou, W; Wang, J; Pan, L; Wagaw, S; Engstrom, K; Kerdesky, FA; Longenecker, K; Judge, R; Qin, W; Imade, HM; Stolarik, D; Beno, DW; Brune, M; Chovan, LE; Sham, HL; Jacobson, P; Link, JT Discovery of adamantane ethers as inhibitors of 11beta-HSD-1: Synthesis and biological evaluation. Bioorg Med Chem Lett17:750-5 (2007) [PubMed] Article Patel, JR; Shuai, Q; Dinges, J; Winn, M; Pliushchev, M; Fung, S; Monzon, K; Chiou, W; Wang, J; Pan, L; Wagaw, S; Engstrom, K; Kerdesky, FA; Longenecker, K; Judge, R; Qin, W; Imade, HM; Stolarik, D; Beno, DW; Brune, M; Chovan, LE; Sham, HL; Jacobson, P; Link, JT Discovery of adamantane ethers as inhibitors of 11beta-HSD-1: Synthesis and biological evaluation. Bioorg Med Chem Lett17:750-5 (2007) [PubMed] Article | ||
| More Info.: | Get all data from this article, Solution Info, Assay Method | ||
| 11-beta-hydroxysteroid dehydrogenase type 2 | |||
| Name: | 11-beta-hydroxysteroid dehydrogenase type 2 | ||
| Synonyms: | 11-DH2 | 11-beta-HSD2 | 11-beta-Hydroxysteroid Dehydrogenase 2 (11-beta-HSD2) | 11-beta-hydroxysteroid dehydrogenase | 11-beta-hydroxysteroid dehydrogenase 2 | 11-beta-hydroxysteroid dehydrogenase type 2 | 11-beta-hydroxysteroid dehydrogenase type 2 (11-beta-HSD2) | Corticosteroid 11-beta-dehydrogenase isozyme 2 | DHI2_HUMAN | HSD11B2 | HSD11K | NAD-dependent 11-beta-hydroxysteroid dehydrogenase | SDR9C3 | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 44141.72 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Purified recombinant human 11beta-HSD2. | ||
| Residue: | 405 | ||
| Sequence: |
| ||
| BDBM13756 | |||
| BDBM13775 | |||
| Name | BDBM13756 | ||
| Synonyms: | (1R,3R,4S,7S)-4-(2-methyl-2-phenoxypropanamido)adamantane-1-carboxamide | adamantane ether 6 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C21H28N2O3 | ||
| Mol. Mass. | 356.4586 | ||
| SMILES | CC(C)(Oc1ccccc1)C(=O)N[C@H]1C2C[C@@H]3C[C@@H]1C[C@](C3)(C2)C(N)=O |r,wU:16.16,18.18,13.13,wD:20.26,TLB:22:20:17:15.14.13,THB:15:16:19:22.14.13,21:16:13:22.19.20,23:20:17:15.14.13,(10.84,-3.12,;11.61,-4.45,;12.7,-3.37,;12.94,-5.22,;14.43,-4.83,;14.43,-3.29,;15.76,-2.52,;17.1,-3.29,;17.1,-4.83,;15.76,-5.6,;10.27,-5.22,;10.27,-6.76,;8.94,-4.45,;7.61,-5.22,;7.61,-6.76,;6.94,-7.93,;4.72,-7.16,;4.72,-5.12,;6.04,-4.32,;4.24,-5.07,;4.24,-6.31,;3.21,-7.32,;6.26,-7.03,;2.91,-5.54,;1.58,-6.31,;2.51,-4.05,)| | ||
| Structure | 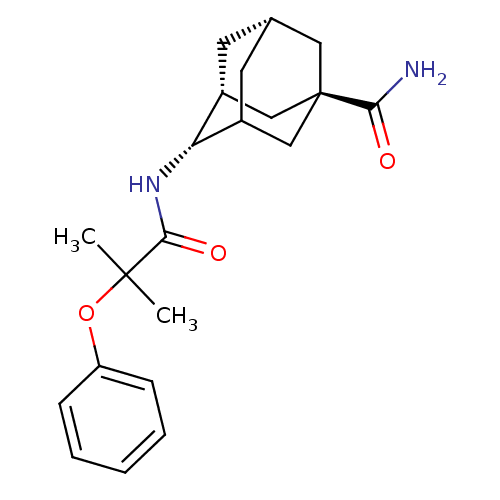
| ||