| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urokinase-type plasminogen activator |
|---|
| Ligand | BDBM16127 |
|---|
| Substrate/Competitor | BDBM14716 |
|---|
| Meas. Tech. | Determination of Inhibitor Potency and Selectivity |
|---|
| pH | 8.1±n/a |
|---|
| Temperature | 310.15±n/a K |
|---|
| Ki | 2300±n/a nM |
|---|
| Km | 90000±n/a nM |
|---|
| Citation |  Fish, PV; Barber, CG; Brown, DG; Butt, R; Collis, MG; Dickinson, RP; Henry, BT; Horne, VA; Huggins, JP; King, E; O'gara, M; McCleverty, D; McIntosh, F; Phillips, C; Webster, R Selective Urokinase-Type Plasminogen Activator Inhibitors. 4. 1-(7-Sulfonamidoisoquinolinyl)guanidines. J Med Chem50:2341-51 (2007) [PubMed] Article Fish, PV; Barber, CG; Brown, DG; Butt, R; Collis, MG; Dickinson, RP; Henry, BT; Horne, VA; Huggins, JP; King, E; O'gara, M; McCleverty, D; McIntosh, F; Phillips, C; Webster, R Selective Urokinase-Type Plasminogen Activator Inhibitors. 4. 1-(7-Sulfonamidoisoquinolinyl)guanidines. J Med Chem50:2341-51 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method |
|---|
| |
| Urokinase-type plasminogen activator |
|---|
| Name: | Urokinase-type plasminogen activator |
|---|
| Synonyms: | 3.4.21.73 | PLAU | U-plasminogen activator | UROK_HUMAN | Urokinase | Urokinase-type plasminogen activator (uPA) | Urokinase-type plasminogen activator chain B | Urokinase-type plasminogen activator long chain A | Urokinase-type plasminogen activator short chain A | Urokinase-type plasminogen activator/surface receptor | uPA |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48528.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00749 |
|---|
| Residue: | 431 |
|---|
| Sequence: | MRALLARLLLCVLVVSDSKGSNELHQVPSNCDCLNGGTCVSNKYFSNIHWCNCPKKFGGQ
HCEIDKSKTCYEGNGHFYRGKASTDTMGRPCLPWNSATVLQQTYHAHRSDALQLGLGKHN
YCRNPDNRRRPWCYVQVGLKLLVQECMVHDCADGKKPSSPPEELKFQCGQKTLRPRFKII
GGEFTTIENQPWFAAIYRRHRGGSVTYVCGGSLISPCWVISATHCFIDYPKKEDYIVYLG
RSRLNSNTQGEMKFEVENLILHKDYSADTLAHHNDIALLKIRSKEGRCAQPSRTIQTICL
PSMYNDPQFGTSCEITGFGKENSTDYLYPEQLKMTVVKLISHRECQQPHYYGSEVTTKML
CAADPQWKTDSCQGDSGGPLVCSLQGRMTLTGIVSWGRGCALKDKPGVYTRVSHFLPWIR
SHTKEENGLAL
|
|
|
|---|
| BDBM16127 |
|---|
| BDBM14716 |
|---|
| Name | BDBM16127 |
|---|
| Synonyms: | 2,2 -methanediylbis(1H-benzimidazole-6-carboximidamide) | 2-[(6-carbamimidoyl-1H-1,3-benzodiazol-2-yl)methyl]-1H-1,3-benzodiazole-6-carboximidamide | AIDS007118 | BABIM | CHEMBL542712 | CHEMBL99951 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H16N8 |
|---|
| Mol. Mass. | 332.3625 |
|---|
| SMILES | NC(=N)c1ccc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2c1 |
|---|
| Structure | 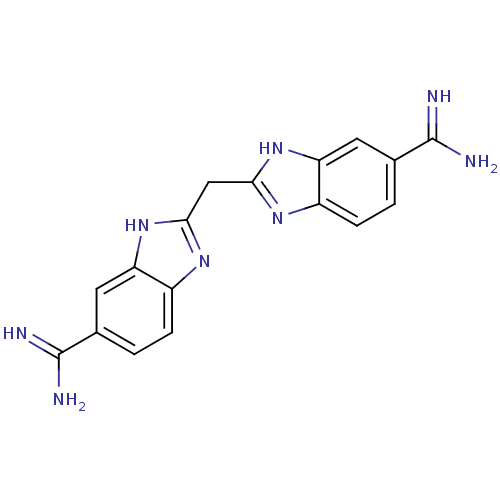
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fish, PV; Barber, CG; Brown, DG; Butt, R; Collis, MG; Dickinson, RP; Henry, BT; Horne, VA; Huggins, JP; King, E; O'gara, M; McCleverty, D; McIntosh, F; Phillips, C; Webster, R Selective Urokinase-Type Plasminogen Activator Inhibitors. 4. 1-(7-Sulfonamidoisoquinolinyl)guanidines. J Med Chem50:2341-51 (2007) [PubMed] Article
Fish, PV; Barber, CG; Brown, DG; Butt, R; Collis, MG; Dickinson, RP; Henry, BT; Horne, VA; Huggins, JP; King, E; O'gara, M; McCleverty, D; McIntosh, F; Phillips, C; Webster, R Selective Urokinase-Type Plasminogen Activator Inhibitors. 4. 1-(7-Sulfonamidoisoquinolinyl)guanidines. J Med Chem50:2341-51 (2007) [PubMed] Article