

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Lysosomal acid glucosylceramidase | ||
| Ligand | BDBM18437 | ||
| Substrate/Competitor | BDBM18429 | ||
| Meas. Tech. | GC Enzyme Assay | ||
| pH | 5.9±n/a | ||
| Temperature | 294.15±n/a K | ||
| Ki | 122000±n/a nM | ||
| Km | 28000±n/a nM | ||
| Citation |  Zheng, W; Padia, J; Urban, DJ; Jadhav, A; Goker-Alpan, O; Simeonov, A; Goldin, E; Auld, D; LaMarca, ME; Inglese, J; Austin, CP; Sidransky, E Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci U S A104:13192-7 (2007) [PubMed] Article Zheng, W; Padia, J; Urban, DJ; Jadhav, A; Goker-Alpan, O; Simeonov, A; Goldin, E; Auld, D; LaMarca, ME; Inglese, J; Austin, CP; Sidransky, E Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci U S A104:13192-7 (2007) [PubMed] Article | ||
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method | ||
| Lysosomal acid glucosylceramidase | |||
| Name: | Lysosomal acid glucosylceramidase | ||
| Synonyms: | Acid beta-glucosidase | Alglucerase | Beta-glucocerebrosidase | Beta-glucocerebrosidase (GC) | D-glucosyl-N-acylsphingosine glucohydrolase | GBA | GBA1 | GBA1_HUMAN | GC | GCase | GLUC | Glucocerebrosidase (GBA) | Glucosylceramidase (GBA) | Glucosylceramidase (GCase) | Glucosylceramidase precursor (Beta-glucocerebrosidase) (Acid beta-glucosidase) (D-glucosyl-N-acylsphingosine glucohydrolase) (Alglucerase) (Imiglucerase) | Imiglucerase | beta-glucocerebrosidase (GCase) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 59724.64 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | The beta-Glu activity was measured with commercially available beta-glucocerebrosidase (Ceredase) as the enzyme source. | ||
| Residue: | 536 | ||
| Sequence: |
| ||
| BDBM18437 | |||
| BDBM18429 | |||
| Name | BDBM18437 | ||
| Synonyms: | Aminoquinoline compound, 22 | N-{2-[(3-chlorophenyl)amino]-4-methylquinolin-6-yl}-4-methylcyclohexane-1-carboxamide | ||
| Type | Small organic molecule | ||
| Emp. Form. | C24H26ClN3O | ||
| Mol. Mass. | 407.936 | ||
| SMILES | CC1CCC(CC1)C(=O)Nc1ccc2nc(Nc3cccc(Cl)c3)cc(C)c2c1 |(-13.5,6.68,;-12.12,6,;-10.84,6.85,;-9.46,6.17,;-9.36,4.63,;-10.64,3.78,;-12.02,4.46,;-8.03,3.86,;-8.03,2.32,;-6.69,4.63,;-5.36,3.86,;-5.36,2.32,;-4.03,1.55,;-2.69,2.32,;-1.36,1.55,;-.02,2.32,;1.31,1.55,;1.28,.01,;-.03,-.8,;.02,-2.34,;1.37,-3.07,;2.68,-2.26,;4.04,-2.99,;2.64,-.72,;-.02,3.86,;-1.36,4.63,;-1.36,6.17,;-2.69,3.86,;-4.03,4.63,)| | ||
| Structure | 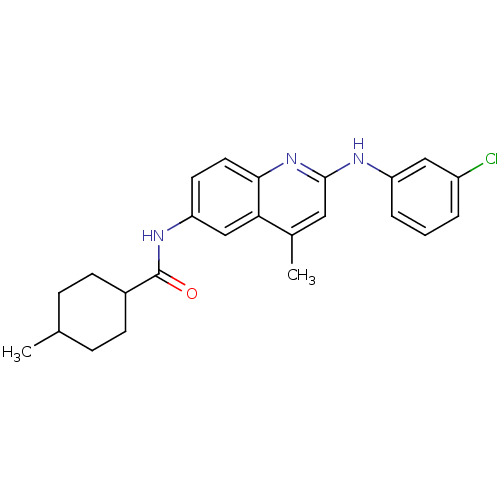
| ||