 Found 1708 hits with Last Name = 'auld' and Initial = 'd'
Found 1708 hits with Last Name = 'auld' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184539

((2S)-2-(6-butyl-2-(4-(4-(trifluoromethyl)phenyl)-1...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H39F3N6O2/c1-5-7-9-23-17-26(36-24(16-20(3)4)27(39)33-14-8-15-40-6-2)37-28(35-23)38-18-25(34-19-38)21-10-12-22(13-11-21)29(30,31)32/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,33,39)(H,35,36,37)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184530
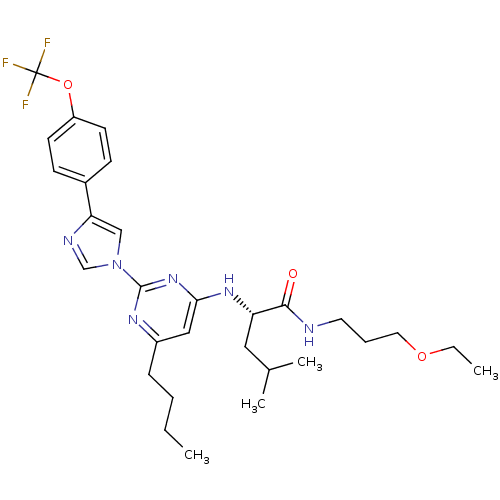
((2S)-2-(6-butyl-2-(4-(4-(trifluoromethoxy)phenyl)-...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H39F3N6O3/c1-5-7-9-22-17-26(36-24(16-20(3)4)27(39)33-14-8-15-40-6-2)37-28(35-22)38-18-25(34-19-38)21-10-12-23(13-11-21)41-29(30,31)32/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,33,39)(H,35,36,37)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18428
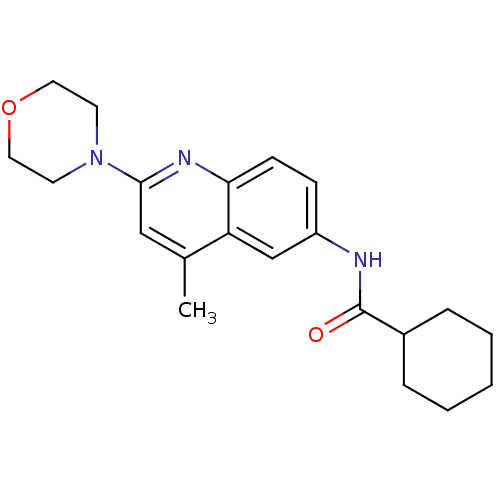
(Aminoquinoline compound, 1 | N-[4-methyl-2-(morpho...)Show InChI InChI=1S/C21H27N3O2/c1-15-13-20(24-9-11-26-12-10-24)23-19-8-7-17(14-18(15)19)22-21(25)16-5-3-2-4-6-16/h7-8,13-14,16H,2-6,9-12H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | -43.2 | 31 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V1a receptor at 5 uM |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184531

((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...)Show SMILES CCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H37F3N6O3/c1-5-8-21-16-25(35-23(15-19(3)4)26(38)32-13-7-14-39-6-2)36-27(34-21)37-17-24(33-18-37)20-9-11-22(12-10-20)40-28(29,30)31/h9-12,16-19,23H,5-8,13-15H2,1-4H3,(H,32,38)(H,34,35,36)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184518

((2S)-2-(6-butyl-2-(4-(4-chlorophenyl)-1H-imidazol-...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H39ClN6O2/c1-5-7-9-23-17-26(33-24(16-20(3)4)27(36)30-14-8-15-37-6-2)34-28(32-23)35-18-25(31-19-35)21-10-12-22(29)13-11-21/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,30,36)(H,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184527

((2S)-2-(6-butyl-2-(4-(4-hydroxyphenyl)-1H-imidazol...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(O)cc1 Show InChI InChI=1S/C28H40N6O3/c1-5-7-9-22-17-26(32-24(16-20(3)4)27(36)29-14-8-15-37-6-2)33-28(31-22)34-18-25(30-19-34)21-10-12-23(35)13-11-21/h10-13,17-20,24,35H,5-9,14-16H2,1-4H3,(H,29,36)(H,31,32,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM100152
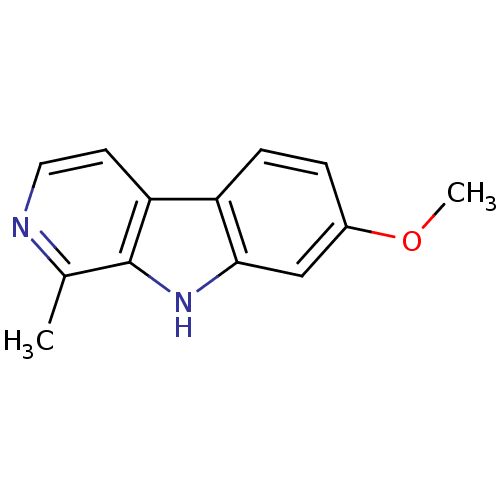
(7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...)Show InChI InChI=1S/C13H12N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-7,15H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Competitive inhibition of GST-tagged DYRK1A (unknown origin) expressed in Escherichia coli BL21(DE3) by Lineweaver-Burk plot analysis in presence of ... |
Bioorg Med Chem Lett 23: 3654-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.096
BindingDB Entry DOI: 10.7270/Q2M90B2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184540
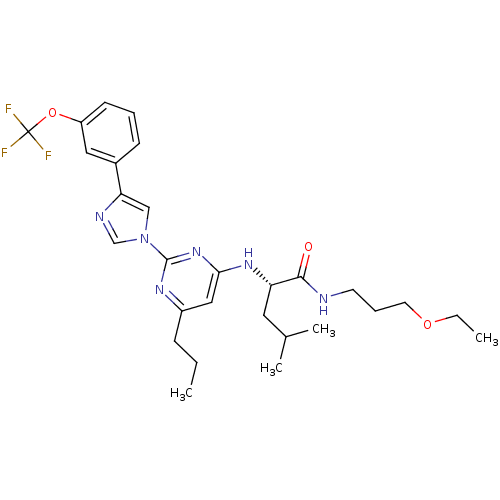
((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...)Show SMILES CCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C28H37F3N6O3/c1-5-9-21-16-25(35-23(14-19(3)4)26(38)32-12-8-13-39-6-2)36-27(34-21)37-17-24(33-18-37)20-10-7-11-22(15-20)40-28(29,30)31/h7,10-11,15-19,23H,5-6,8-9,12-14H2,1-4H3,(H,32,38)(H,34,35,36)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184536
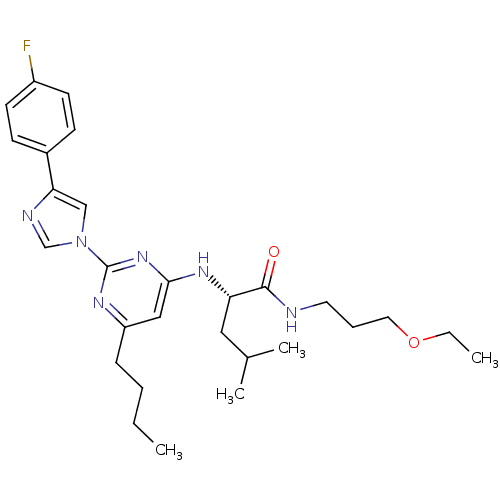
((2S)-2-(6-butyl-2-(4-(4-fluorophenyl)-1H-imidazol-...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(F)cc1 Show InChI InChI=1S/C28H39FN6O2/c1-5-7-9-23-17-26(33-24(16-20(3)4)27(36)30-14-8-15-37-6-2)34-28(32-23)35-18-25(31-19-35)21-10-12-22(29)13-11-21/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,30,36)(H,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184535
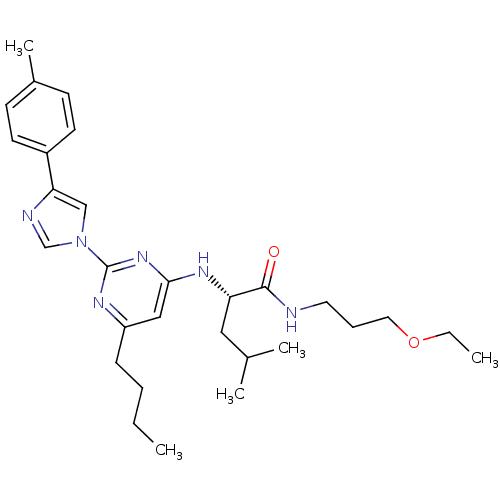
((2S)-2-(6-butyl-2-(4-p-tolyl-1H-imidazol-1-yl)pyri...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(C)cc1 Show InChI InChI=1S/C29H42N6O2/c1-6-8-10-24-18-27(33-25(17-21(3)4)28(36)30-15-9-16-37-7-2)34-29(32-24)35-19-26(31-20-35)23-13-11-22(5)12-14-23/h11-14,18-21,25H,6-10,15-17H2,1-5H3,(H,30,36)(H,32,33,34)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184521
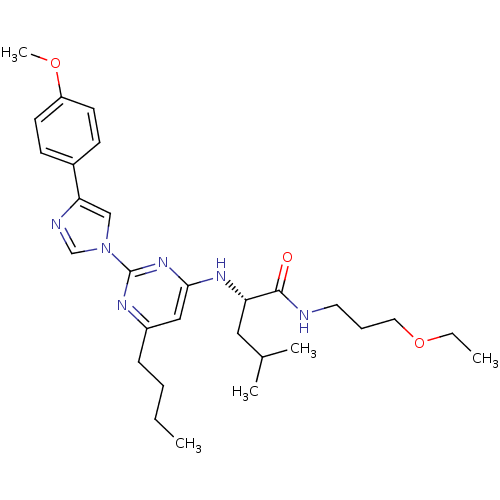
((2S)-2-(6-butyl-2-(4-(4-methoxyphenyl)-1H-imidazol...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(OC)cc1 Show InChI InChI=1S/C29H42N6O3/c1-6-8-10-23-18-27(33-25(17-21(3)4)28(36)30-15-9-16-38-7-2)34-29(32-23)35-19-26(31-20-35)22-11-13-24(37-5)14-12-22/h11-14,18-21,25H,6-10,15-17H2,1-5H3,(H,30,36)(H,32,33,34)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184523
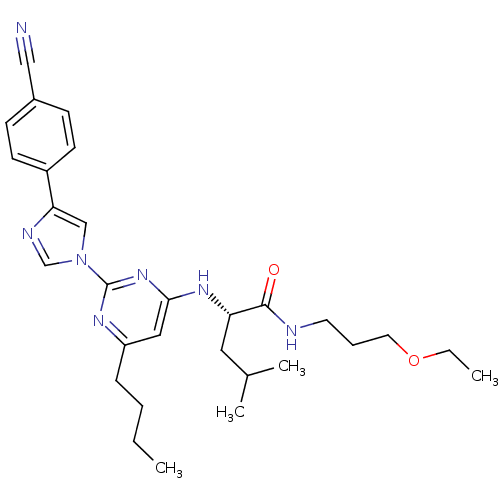
((2S)-2-(6-butyl-2-(4-(4-cyanophenyl)-1H-imidazol-1...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C29H39N7O2/c1-5-7-9-24-17-27(34-25(16-21(3)4)28(37)31-14-8-15-38-6-2)35-29(33-24)36-19-26(32-20-36)23-12-10-22(18-30)11-13-23/h10-13,17,19-21,25H,5-9,14-16H2,1-4H3,(H,31,37)(H,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184517

((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-methyl-2-(4-...)Show SMILES CCOCCCNC(=O)[C@H](CC(C)C)Nc1cc(C)nc(n1)-n1cnc(c1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C26H33F3N6O3/c1-5-37-12-6-11-30-24(36)21(13-17(2)3)33-23-14-18(4)32-25(34-23)35-15-22(31-16-35)19-7-9-20(10-8-19)38-26(27,28)29/h7-10,14-17,21H,5-6,11-13H2,1-4H3,(H,30,36)(H,32,33,34)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184537

((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...)Show SMILES CCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C28H37F3N6O3/c1-5-10-20-16-25(35-22(15-19(3)4)26(38)32-13-9-14-39-6-2)36-27(34-20)37-17-23(33-18-37)21-11-7-8-12-24(21)40-28(29,30)31/h7-8,11-12,16-19,22H,5-6,9-10,13-15H2,1-4H3,(H,32,38)(H,34,35,36)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18438

(4-benzenesulfonamido-N-(5-ethyl-1,3,4-thiadiazol-2...)Show SMILES CCc1nnc(NC(=O)c2ccc(NS(=O)(=O)c3ccccc3)cc2)s1 Show InChI InChI=1S/C17H16N4O3S2/c1-2-15-19-20-17(25-15)18-16(22)12-8-10-13(11-9-12)21-26(23,24)14-6-4-3-5-7-14/h3-11,21H,2H2,1H3,(H,18,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | -41.0 | 103 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
ATP-dependent 6-phosphofructokinase
(Trypanosoma brucei) | BDBM50446088
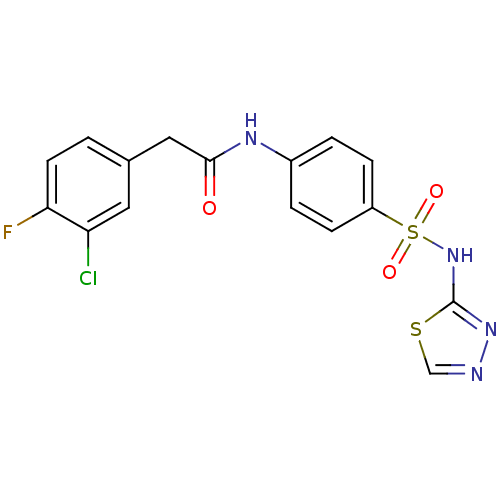
(CHEMBL3108870)Show SMILES Fc1ccc(CC(=O)Nc2ccc(cc2)S(=O)(=O)Nc2nncs2)cc1Cl Show InChI InChI=1S/C16H12ClFN4O3S2/c17-13-7-10(1-6-14(13)18)8-15(23)20-11-2-4-12(5-3-11)27(24,25)22-16-21-19-9-26-16/h1-7,9H,8H2,(H,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Competitive inhibition Trypanosoma brucei PFK using fructose-6-phosphate as substrate by Line-weaver Burk plot analysis |
ACS Med Chem Lett 5: 12-7 (2014)
Article DOI: 10.1021/ml400259d
BindingDB Entry DOI: 10.7270/Q2X34ZX7 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18431

(Aminoquinoline compound, 16 | N-[4-methyl-2-(morph...)Show SMILES CCCC1CCC(CC1)C(=O)Nc1ccc2nc(cc(C)c2c1)N1CCOCC1 |(-16.03,6.94,;-14.69,6.17,;-13.36,6.94,;-12.03,6.17,;-10.69,6.94,;-9.36,6.17,;-9.36,4.63,;-10.69,3.86,;-12.03,4.63,;-8.03,3.86,;-8.03,2.32,;-6.69,4.63,;-5.36,3.86,;-5.36,2.32,;-4.03,1.55,;-2.69,2.32,;-1.36,1.55,;-.02,2.32,;-.02,3.86,;-1.36,4.63,;-1.36,6.17,;-2.69,3.86,;-4.03,4.63,;1.31,1.55,;1.31,.01,;2.64,-.76,;3.98,.01,;3.98,1.55,;2.64,2.32,)| Show InChI InChI=1S/C24H33N3O2/c1-3-4-18-5-7-19(8-6-18)24(28)25-20-9-10-22-21(16-20)17(2)15-23(26-22)27-11-13-29-14-12-27/h9-10,15-16,18-19H,3-8,11-14H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | -40.9 | 133 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18430
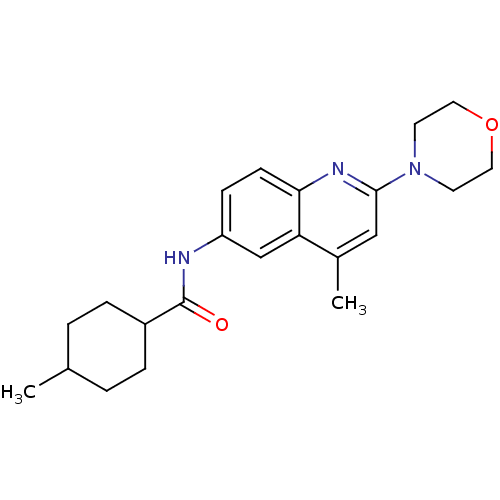
(4-methyl-N-[4-methyl-2-(morpholin-4-yl)quinolin-6-...)Show SMILES CC1CCC(CC1)C(=O)Nc1ccc2nc(cc(C)c2c1)N1CCOCC1 |(-13.36,6.94,;-12.03,6.17,;-10.69,6.94,;-9.36,6.17,;-9.36,4.63,;-10.69,3.86,;-12.03,4.63,;-8.03,3.86,;-8.03,2.32,;-6.69,4.63,;-5.36,3.86,;-5.36,2.32,;-4.03,1.55,;-2.69,2.32,;-1.36,1.55,;-.02,2.32,;-.02,3.86,;-1.36,4.63,;-1.36,6.17,;-2.69,3.86,;-4.03,4.63,;1.31,1.55,;1.31,.01,;2.64,-.76,;3.98,.01,;3.98,1.55,;2.64,2.32,)| Show InChI InChI=1S/C22H29N3O2/c1-15-3-5-17(6-4-15)22(26)23-18-7-8-20-19(14-18)16(2)13-21(24-20)25-9-11-27-12-10-25/h7-8,13-15,17H,3-6,9-12H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | -40.8 | 63 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184516

((2S)-N-(3-ethoxypropyl)-2-(6-ethyl-2-(4-(4-(triflu...)Show SMILES CCOCCCNC(=O)[C@H](CC(C)C)Nc1cc(CC)nc(n1)-n1cnc(c1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H35F3N6O3/c1-5-20-15-24(34-22(14-18(3)4)25(37)31-12-7-13-38-6-2)35-26(33-20)36-16-23(32-17-36)19-8-10-21(11-9-19)39-27(28,29)30/h8-11,15-18,22H,5-7,12-14H2,1-4H3,(H,31,37)(H,33,34,35)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184524

((2S)-2-(2-(1H-imidazol-1-yl)-6-(octylthio)pyrimidi...)Show SMILES CCCCCCCCSc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1ccnc1 Show InChI InChI=1S/C26H44N6O2S/c1-5-7-8-9-10-11-17-35-24-19-23(30-26(31-24)32-15-14-27-20-32)29-22(18-21(3)4)25(33)28-13-12-16-34-6-2/h14-15,19-22H,5-13,16-18H2,1-4H3,(H,28,33)(H,29,30,31)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184532
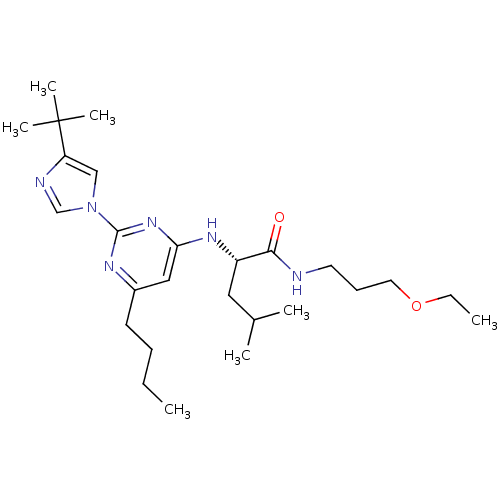
((2S)-2-(6-butyl-2-(4-tert-butyl-1H-imidazol-1-yl)p...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)C(C)(C)C Show InChI InChI=1S/C26H44N6O2/c1-8-10-12-20-16-23(31-25(29-20)32-17-22(28-18-32)26(5,6)7)30-21(15-19(3)4)24(33)27-13-11-14-34-9-2/h16-19,21H,8-15H2,1-7H3,(H,27,33)(H,29,30,31)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184541

((2S)-2-(6-butyl-2-(4-methyl-1H-imidazol-1-yl)pyrim...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(C)c1 Show InChI InChI=1S/C23H38N6O2/c1-6-8-10-19-14-21(28-23(26-19)29-15-18(5)25-16-29)27-20(13-17(3)4)22(30)24-11-9-12-31-7-2/h14-17,20H,6-13H2,1-5H3,(H,24,30)(H,26,27,28)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184542
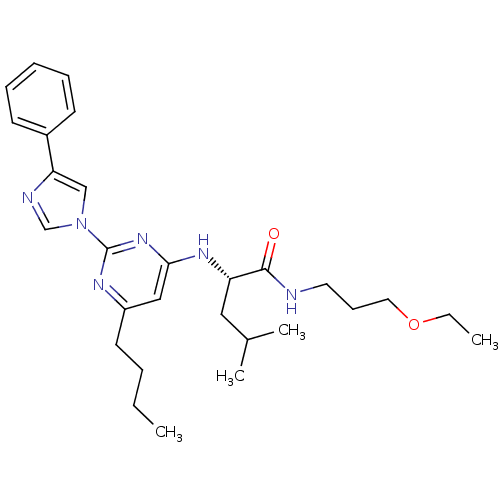
((2S)-2-(6-butyl-2-(4-phenyl-1H-imidazol-1-yl)pyrim...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccccc1 Show InChI InChI=1S/C28H40N6O2/c1-5-7-14-23-18-26(32-24(17-21(3)4)27(35)29-15-11-16-36-6-2)33-28(31-23)34-19-25(30-20-34)22-12-9-8-10-13-22/h8-10,12-13,18-21,24H,5-7,11,14-17H2,1-4H3,(H,29,35)(H,31,32,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18439
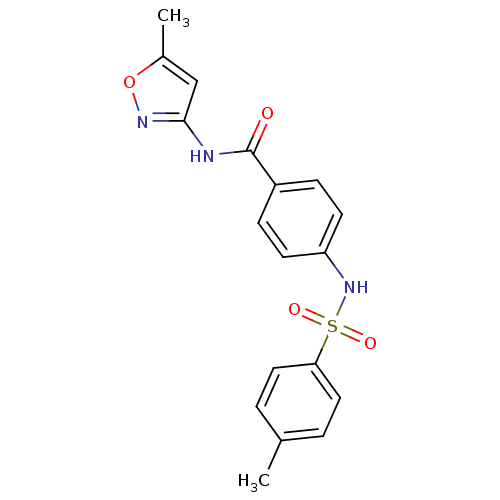
(N-(5-methyl-1,2-oxazol-3-yl)-4-[(4-methylbenzene)s...)Show SMILES Cc1cc(NC(=O)c2ccc(NS(=O)(=O)c3ccc(C)cc3)cc2)no1 Show InChI InChI=1S/C18H17N3O4S/c1-12-3-9-16(10-4-12)26(23,24)21-15-7-5-14(6-8-15)18(22)19-17-11-13(2)25-20-17/h3-11,21H,1-2H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 102 | -39.4 | 168 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18433
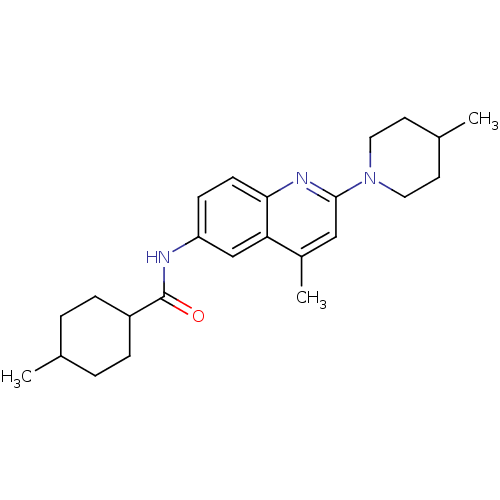
(4-methyl-N-[4-methyl-2-(4-methylpiperidin-1-yl)qui...)Show SMILES CC1CCC(CC1)C(=O)Nc1ccc2nc(cc(C)c2c1)N1CCC(C)CC1 |(-13.5,6.68,;-12.12,6,;-10.84,6.85,;-9.46,6.17,;-9.36,4.63,;-10.64,3.78,;-12.02,4.46,;-8.03,3.86,;-8.03,2.32,;-6.69,4.63,;-5.36,3.86,;-5.36,2.32,;-4.03,1.55,;-2.69,2.32,;-1.36,1.55,;-.02,2.32,;-.02,3.86,;-1.36,4.63,;-1.36,6.17,;-2.69,3.86,;-4.03,4.63,;1.31,1.55,;1.31,.01,;2.64,-.76,;3.98,.01,;5.31,-.76,;3.98,1.55,;2.64,2.32,)| Show InChI InChI=1S/C24H33N3O/c1-16-4-6-19(7-5-16)24(28)25-20-8-9-22-21(15-20)18(3)14-23(26-22)27-12-10-17(2)11-13-27/h8-9,14-17,19H,4-7,10-13H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | -39.0 | 268 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184519

((2S)-2-(2-(1H-imidazol-1-yl)-6-octylpyrimidin-4-yl...)Show SMILES CCCCCCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1ccnc1 Show InChI InChI=1S/C26H44N6O2/c1-5-7-8-9-10-11-13-22-19-24(31-26(29-22)32-16-15-27-20-32)30-23(18-21(3)4)25(33)28-14-12-17-34-6-2/h15-16,19-21,23H,5-14,17-18H2,1-4H3,(H,28,33)(H,29,30,31)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18432
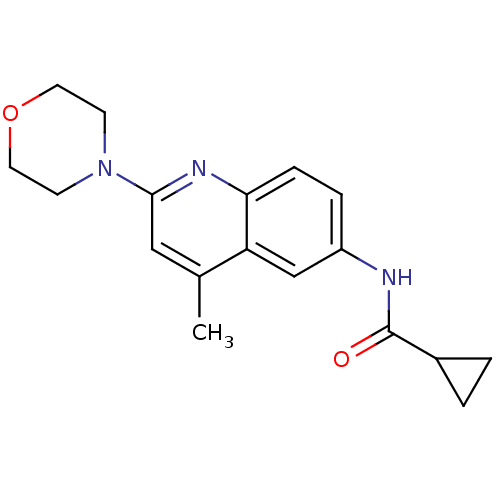
(Aminoquinoline compound, 17 | N-[4-methyl-2-(morph...)Show InChI InChI=1S/C18H21N3O2/c1-12-10-17(21-6-8-23-9-7-21)20-16-5-4-14(11-15(12)16)19-18(22)13-2-3-13/h4-5,10-11,13H,2-3,6-9H2,1H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | -39.0 | 183 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184526
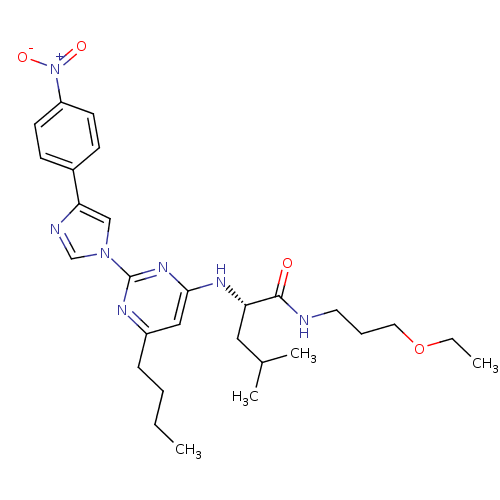
((2S)-2-(6-butyl-2-(4-(4-nitrophenyl)-1H-imidazol-1...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C28H39N7O4/c1-5-7-9-22-17-26(32-24(16-20(3)4)27(36)29-14-8-15-39-6-2)33-28(31-22)34-18-25(30-19-34)21-10-12-23(13-11-21)35(37)38/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,29,36)(H,31,32,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM100152

(7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...)Show InChI InChI=1S/C13H12N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-7,15H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center
Curated by ChEMBL
| Assay Description
Competitive inhibition of GST-tagged DYRK1B (unknown origin) expressed in Escherichia coli BL21(DE3) by Lineweaver-Burk plot analysis in presence of ... |
Bioorg Med Chem Lett 23: 3654-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.096
BindingDB Entry DOI: 10.7270/Q2M90B2B |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18434

(4-methyl-N-[4-methyl-2-(piperidin-1-yl)quinolin-6-...)Show SMILES CC1CCC(CC1)C(=O)Nc1ccc2nc(cc(C)c2c1)N1CCCCC1 |(-13.5,6.68,;-12.12,6,;-10.84,6.85,;-9.46,6.17,;-9.36,4.63,;-10.64,3.78,;-12.02,4.46,;-8.03,3.86,;-8.03,2.32,;-6.69,4.63,;-5.36,3.86,;-5.36,2.32,;-4.03,1.55,;-2.69,2.32,;-1.36,1.55,;-.02,2.32,;-.02,3.86,;-1.36,4.63,;-1.36,6.17,;-2.69,3.86,;-4.03,4.63,;1.31,1.55,;1.31,.01,;2.64,-.76,;3.98,.01,;3.98,1.55,;2.64,2.32,)| Show InChI InChI=1S/C23H31N3O/c1-16-6-8-18(9-7-16)23(27)24-19-10-11-21-20(15-19)17(2)14-22(25-21)26-12-4-3-5-13-26/h10-11,14-16,18H,3-9,12-13H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 184 | -37.9 | 452 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184529
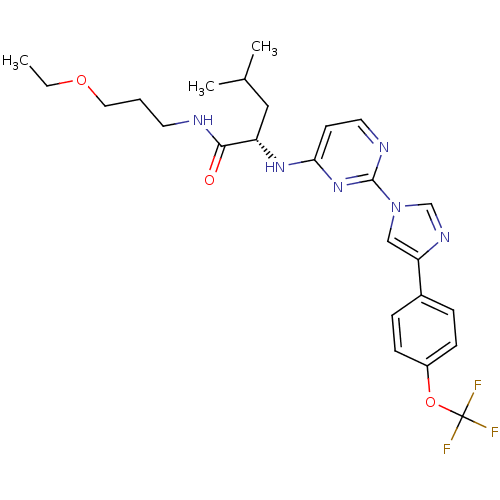
((2S)-N-(3-ethoxypropyl)-4-methyl-2-(2-(4-(4-(trifl...)Show SMILES CCOCCCNC(=O)[C@H](CC(C)C)Nc1ccnc(n1)-n1cnc(c1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C25H31F3N6O3/c1-4-36-13-5-11-29-23(35)20(14-17(2)3)32-22-10-12-30-24(33-22)34-15-21(31-16-34)18-6-8-19(9-7-18)37-25(26,27)28/h6-10,12,15-17,20H,4-5,11,13-14H2,1-3H3,(H,29,35)(H,30,32,33)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
ATP-dependent 6-phosphofructokinase
(Trypanosoma brucei) | BDBM50446088

(CHEMBL3108870)Show SMILES Fc1ccc(CC(=O)Nc2ccc(cc2)S(=O)(=O)Nc2nncs2)cc1Cl Show InChI InChI=1S/C16H12ClFN4O3S2/c17-13-7-10(1-6-14(13)18)8-15(23)20-11-2-4-12(5-3-11)27(24,25)22-16-21-19-9-26-16/h1-7,9H,8H2,(H,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Mixed type inhibition Trypanosoma brucei PFK using ATP as substrate by Line-weaver Burk plot analysis |
ACS Med Chem Lett 5: 12-7 (2014)
Article DOI: 10.1021/ml400259d
BindingDB Entry DOI: 10.7270/Q2X34ZX7 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184538
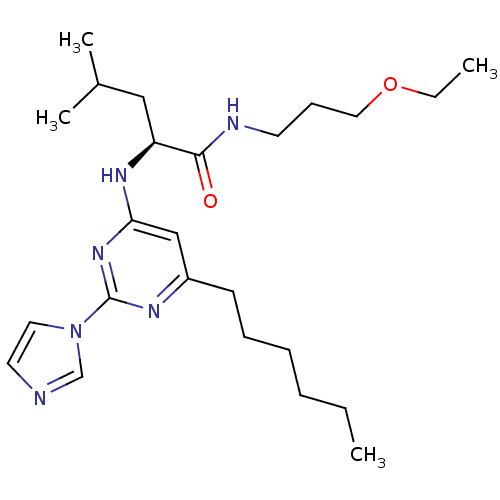
((2S)-N-(3-ethoxypropyl)-2-(6-hexyl-2-(1H-imidazol-...)Show SMILES CCCCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1ccnc1 Show InChI InChI=1S/C24H40N6O2/c1-5-7-8-9-11-20-17-22(29-24(27-20)30-14-13-25-18-30)28-21(16-19(3)4)23(31)26-12-10-15-32-6-2/h13-14,17-19,21H,5-12,15-16H2,1-4H3,(H,26,31)(H,27,28,29)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18447

(2-({4-[(5-chloro-2-methoxyphenyl)amino]-6-(pyrroli...)Show InChI InChI=1S/C16H21ClN6O2/c1-25-13-5-4-11(17)10-12(13)19-15-20-14(18-6-9-24)21-16(22-15)23-7-2-3-8-23/h4-5,10,24H,2-3,6-9H2,1H3,(H2,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | -36.6 | 430 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V2 receptor |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18435

(Aminoquinoline compound, 20 | N-[2-(diethylamino)-...)Show SMILES CCN(CC)c1cc(C)c2cc(NC(=O)C3CCC(C)CC3)ccc2n1 |(2.64,-.76,;1.31,.01,;1.31,1.55,;2.64,2.32,;3.98,1.55,;-.02,2.32,;-.02,3.86,;-1.36,4.63,;-1.36,6.17,;-2.69,3.86,;-4.03,4.63,;-5.36,3.86,;-6.69,4.63,;-8.03,3.86,;-8.03,2.32,;-9.36,4.63,;-9.46,6.17,;-10.84,6.85,;-12.12,6,;-13.5,6.68,;-12.02,4.46,;-10.64,3.78,;-5.36,2.32,;-4.03,1.55,;-2.69,2.32,;-1.36,1.55,)| Show InChI InChI=1S/C22H31N3O/c1-5-25(6-2)21-13-16(4)19-14-18(11-12-20(19)24-21)23-22(26)17-9-7-15(3)8-10-17/h11-15,17H,5-10H2,1-4H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 514 | -35.4 | 1.06E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18443
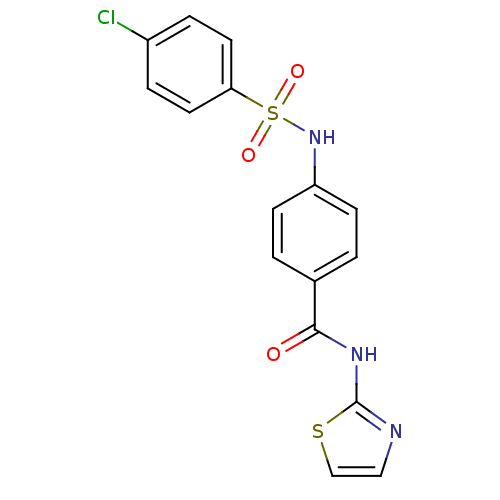
(4-[(4-chlorobenzene)sulfonamido]-N-(1,3-thiazol-2-...)Show SMILES Clc1ccc(cc1)S(=O)(=O)Nc1ccc(cc1)C(=O)Nc1nccs1 Show InChI InChI=1S/C16H12ClN3O3S2/c17-12-3-7-14(8-4-12)25(22,23)20-13-5-1-11(2-6-13)15(21)19-16-18-9-10-24-16/h1-10,20H,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 556 | -35.2 | 1.29E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184528
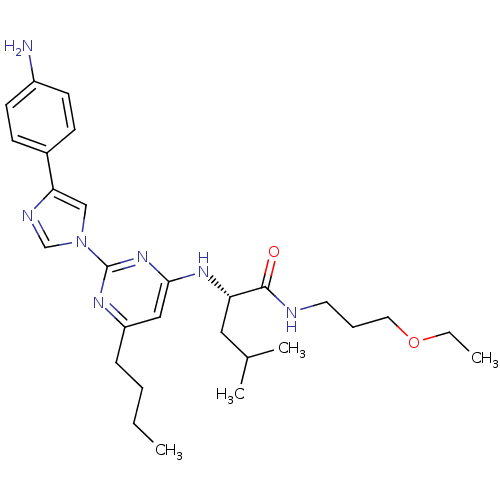
((2S)-2-(2-(4-(4-aminophenyl)-1H-imidazol-1-yl)-6-b...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(N)cc1 Show InChI InChI=1S/C28H41N7O2/c1-5-7-9-23-17-26(33-24(16-20(3)4)27(36)30-14-8-15-37-6-2)34-28(32-23)35-18-25(31-19-35)21-10-12-22(29)13-11-21/h10-13,17-20,24H,5-9,14-16,29H2,1-4H3,(H,30,36)(H,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184525
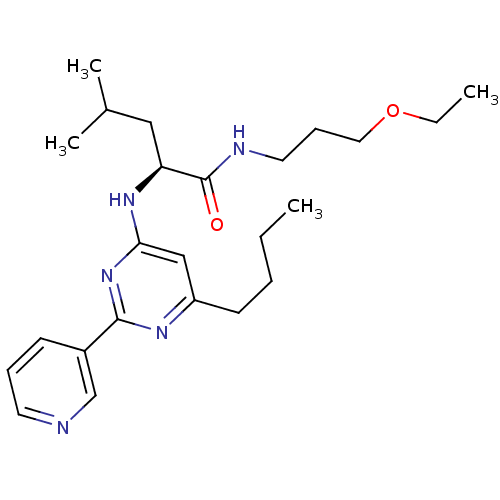
((2S)-2-(6-butyl-2-(pyridin-3-yl)pyrimidin-4-ylamin...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-c1cccnc1 Show InChI InChI=1S/C24H37N5O2/c1-5-7-11-20-16-22(29-23(27-20)19-10-8-12-25-17-19)28-21(15-18(3)4)24(30)26-13-9-14-31-6-2/h8,10,12,16-18,21H,5-7,9,11,13-15H2,1-4H3,(H,26,30)(H,27,28,29)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18436
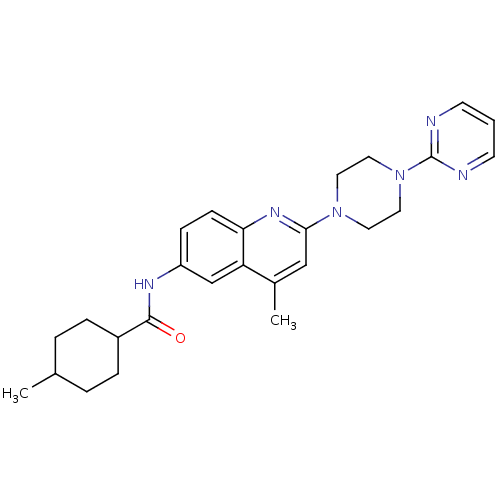
(4-methyl-N-{4-methyl-2-[4-(pyrimidin-2-yl)piperazi...)Show SMILES CC1CCC(CC1)C(=O)Nc1ccc2nc(cc(C)c2c1)N1CCN(CC1)c1ncccn1 |(-13.5,6.68,;-12.12,6,;-10.84,6.85,;-9.46,6.17,;-9.36,4.63,;-10.64,3.78,;-12.02,4.46,;-8.03,3.86,;-8.03,2.32,;-6.69,4.63,;-5.36,3.86,;-5.36,2.32,;-4.03,1.55,;-2.69,2.32,;-1.36,1.55,;-.02,2.32,;-.02,3.86,;-1.36,4.63,;-1.36,6.17,;-2.69,3.86,;-4.03,4.63,;1.31,1.55,;2.66,2.3,;3.98,1.5,;3.95,-.04,;2.6,-.78,;1.28,.01,;5.27,-.84,;6.57,-.02,;7.93,-.75,;7.98,-2.29,;6.67,-3.1,;5.32,-2.37,)| Show InChI InChI=1S/C26H32N6O/c1-18-4-6-20(7-5-18)25(33)29-21-8-9-23-22(17-21)19(2)16-24(30-23)31-12-14-32(15-13-31)26-27-10-3-11-28-26/h3,8-11,16-18,20H,4-7,12-15H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 975 | -33.8 | 2.45E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
Uridine-cytidine kinase 2
(Homo sapiens) | BDBM50522195

(CHEMBL1592234)Show SMILES Cc1ccc(cc1)-c1nc2Oc3c(C)cccc3Cc2c(SCC(=O)Nc2cccc(c2)C(O)=O)n1 Show InChI InChI=1S/C28H23N3O4S/c1-16-9-11-18(12-10-16)25-30-26-22(14-19-6-3-5-17(2)24(19)35-26)27(31-25)36-15-23(32)29-21-8-4-7-20(13-21)28(33)34/h3-13H,14-15H2,1-2H3,(H,29,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of C-terminally His6-tagged human UCK2 expressed in Escherichia coli BL21(DE3) cells using uridine, phosphoenolpyruvate, N... |
Bioorg Med Chem Lett 29: 2559-2564 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.010
BindingDB Entry DOI: 10.7270/Q2DZ0CQZ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184533
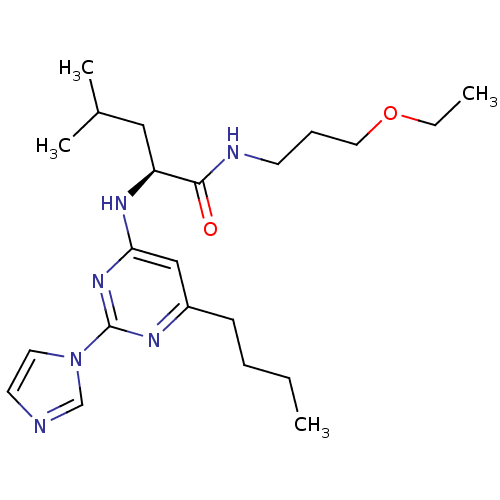
((2S)-2-(6-butyl-2-(1H-imidazol-1-yl)pyrimidin-4-yl...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1ccnc1 Show InChI InChI=1S/C22H36N6O2/c1-5-7-9-18-15-20(27-22(25-18)28-12-11-23-16-28)26-19(14-17(3)4)21(29)24-10-8-13-30-6-2/h11-12,15-17,19H,5-10,13-14H2,1-4H3,(H,24,29)(H,25,26,27)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18448

(2-({4-[(3-methylphenyl)amino]-6-(pyrrolidin-1-yl)-...)Show InChI InChI=1S/C16H22N6O/c1-12-5-4-6-13(11-12)18-15-19-14(17-7-10-23)20-16(21-15)22-8-2-3-9-22/h4-6,11,23H,2-3,7-10H2,1H3,(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.78E+3 | -31.3 | 4.31E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
Uridine-cytidine kinase 2
(Homo sapiens) | BDBM50522195

(CHEMBL1592234)Show SMILES Cc1ccc(cc1)-c1nc2Oc3c(C)cccc3Cc2c(SCC(=O)Nc2cccc(c2)C(O)=O)n1 Show InChI InChI=1S/C28H23N3O4S/c1-16-9-11-18(12-10-16)25-30-26-22(14-19-6-3-5-17(2)24(19)35-26)27(31-25)36-15-23(32)29-21-8-4-7-20(13-21)28(33)34/h3-13H,14-15H2,1-2H3,(H,29,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of C-terminally His6-tagged human UCK2 expressed in Escherichia coli BL21(DE3) cells using phosphoenolpyruvate, NADH and v... |
Bioorg Med Chem Lett 29: 2559-2564 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.010
BindingDB Entry DOI: 10.7270/Q2DZ0CQZ |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM18449
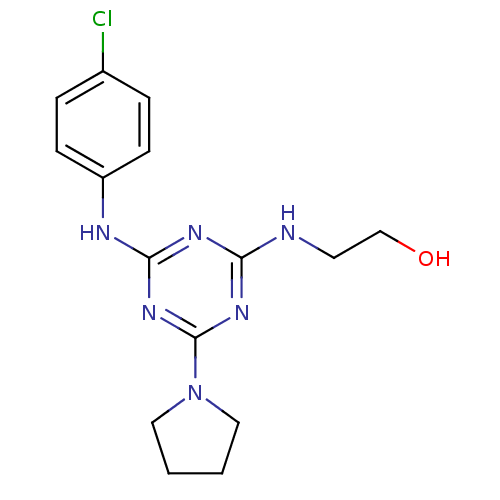
(2-({4-[(4-chlorophenyl)amino]-6-(pyrrolidin-1-yl)-...)Show InChI InChI=1S/C15H19ClN6O/c16-11-3-5-12(6-4-11)18-14-19-13(17-7-10-23)20-15(21-14)22-8-1-2-9-22/h3-6,23H,1-2,7-10H2,(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.23E+3 | -30.3 | 7.73E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH
| Assay Description
Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... |
Proc Natl Acad Sci U S A 104: 13192-7 (2007)
Article DOI: 10.1073/pnas.0705637104
BindingDB Entry DOI: 10.7270/Q2V1232M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184515
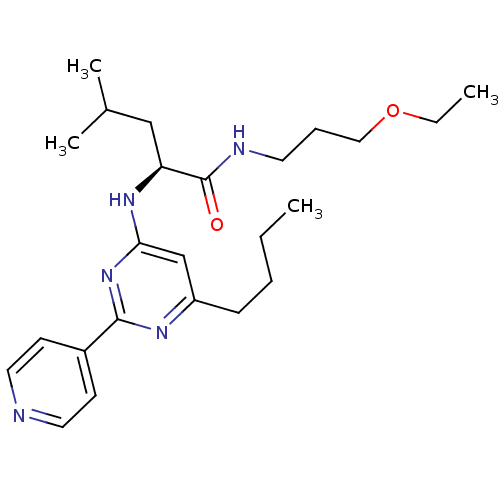
((S)-2-(6-butyl-2-(pyridin-4-yl)pyrimidin-4-ylamino...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-c1ccncc1 Show InChI InChI=1S/C24H37N5O2/c1-5-7-9-20-17-22(29-23(27-20)19-10-13-25-14-11-19)28-21(16-18(3)4)24(30)26-12-8-15-31-6-2/h10-11,13-14,17-18,21H,5-9,12,15-16H2,1-4H3,(H,26,30)(H,27,28,29)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data