| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Gag-Pol polyprotein [A364V] |
|---|
| Ligand | BDBM372369 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | HIV Cell Culture Assay |
|---|
| EC50 | >50±n/a nM |
|---|
| Citation |  Sit, S; Chen, Y; Chen, J; Swidorski, J; Venables, BL; Sin, N; Meanwell, NA; Regueiro-Ren, A; Hartz, RA; Xu, L; Liu, Z Triterpenoids with HIV maturation inhibitory activity US Patent US10245275 Publication Date 4/2/2019 Sit, S; Chen, Y; Chen, J; Swidorski, J; Venables, BL; Sin, N; Meanwell, NA; Regueiro-Ren, A; Hartz, RA; Xu, L; Liu, Z Triterpenoids with HIV maturation inhibitory activity US Patent US10245275 Publication Date 4/2/2019 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Gag-Pol polyprotein [A364V] |
|---|
| Name: | Gag-Pol polyprotein [A364V] |
|---|
| Synonyms: | HIV-1 NL4-3 | HIV-1 gap A364V | POL_HV1N5 | gag-pol |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 161848.10 |
|---|
| Organism: | Human immunodeficiency virus type 1 group M subtype B (isolate NY5) (HIV-1) |
|---|
| Description: | P12497[A364V] |
|---|
| Residue: | 1435 |
|---|
| Sequence: | MGARASVLSGGELDKWEKIRLRPGGKKQYKLKHIVWASRELERFAVNPGLLETSEGCRQI
LGQLQPSLQTGSEELRSLYNTIAVLYCVHQRIDVKDTKEALDKIEEEQNKSKKKAQQAAA
DTGNNSQVSQNYPIVQNLQGQMVHQAISPRTLNAWVKVVEEKAFSPEVIPMFSALSEGAT
PQDLNTMLNTVGGHQAAMQMLKETINEEAAEWDRLHPVHAGPIAPGQMREPRGSDIAGTT
STLQEQIGWMTHNPPIPVGEIYKRWIILGLNKIVRMYSPTSILDIRQGPKEPFRDYVDRF
YKTLRAEQASQEVKNWMTETLLVQNANPDCKTILKALGPGATLEEMMTACQGVGGPGHKA
RVLVEAMSQVTNPATIMIQKGNFRNQRKTVKCFNCGKEGHIAKNCRAPRKKGCWKCGKEG
HQMKDCTERQANFLREDLAFPQGKAREFSSEQTRANSPTRRELQVWGRDNNSLSEAGADR
QGTVSFSFPQITLWQRPLVTIKIGGQLKEALLDTGADDTVLEEMNLPGRWKPKMIGGIGG
FIKVRQYDQILIEICGHKAIGTVLVGPTPVNIIGRNLLTQIGCTLNFPISPIETVPVKLK
PGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPVFAIKKKDSTKWRK
LVDFRELNKRTQDFWEVQLGIPHPAGLKQKKSVTVLDVGDAYFSVPLDKDFRKYTAFTIP
SINNETPGIRYQYNVLPQGWKGSPAIFQCSMTKILEPFRKQNPDIVIYQYMDDLYVGSDL
EIGQHRTKIEELRQHLLRWGFTTPDKKHQKEPPFLWMGYELHPDKWTVQPIVLPEKDSWT
VNDIQKLVGKLNWASQIYAGIKVRQLCKLLRGTKALTEVVPLTEEAELELAENREILKEP
VHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLKTGKYARMKGAHTNDVKQLTEAVQ
KIATESIVIWGKTPKFKLPIQKETWEAWWTEYWQATWIPEWEFVNTPPLVKLWYQLEKEP
IIGAETFYVDGAANRETKLGKAGYVTDRGRQKVVPLTDTTNQKTELQAIHLALQDSGLEV
NIVTDSQYALGIIQAQPDKSESELVSQIIEQLIKKEKVYLAWVPAHKGIGGNEQVDGLVS
AGIRKVLFLDGIDKAQEEHEKYHSNWRAMASDFNLPPVVAKEIVASCDKCQLKGEAMHGQ
VDCSPGIWQLDCTHLEGKVILVAVHVASGYIEAEVIPAETGQETAYFLLKLAGRWPVKTV
HTDNGSNFTSTTVKAACWWAGIKQEFGIPYNPQSQGVIESMNKELKKIIGQVRDQAEHLK
TAVQMAVFIHNFKRKGGIGGYSAGERIVDIIATDIQTKELQKQITKIQNFRVYYRDSRDP
VWKGPAKLLWKGEGAVVIQDNSDIKVVPRRKAKIIRDYGKQMAGDDCVASRQDED
|
|
|
|---|
| BDBM372369 |
|---|
| n/a |
|---|
| Name | BDBM372369 |
|---|
| Synonyms: | Preparation of (1R)-4-((1R,3aS,5aR,5bR,7R,7aR,11aS,11bR,13aR,13bR)-3a-((2-(2,6-dimethyl-1,1-dioxidothiomorpholino)ethyl)amino)-5a,5b, 8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl)-1-(fluoromethyl)cyclohex-3-enecarboxylic acid | US10245275, Example 24 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C45H71FN2O4S |
|---|
| Mol. Mass. | 755.12 |
|---|
| SMILES | CC1CN(CCN[C@]23CC[C@H]([C@@H]2[C@H]2CC[C@@H]4[C@@]5(C)CC=C(C6=CC[C@](CF)(CC6)OC=O)C(C)(C)[C@@H]5CC[C@@]4(C)[C@]2(C)CC3)C(C)=C)CC(C)S1(=O)=O |r,t:20,22| |
|---|
| Structure | 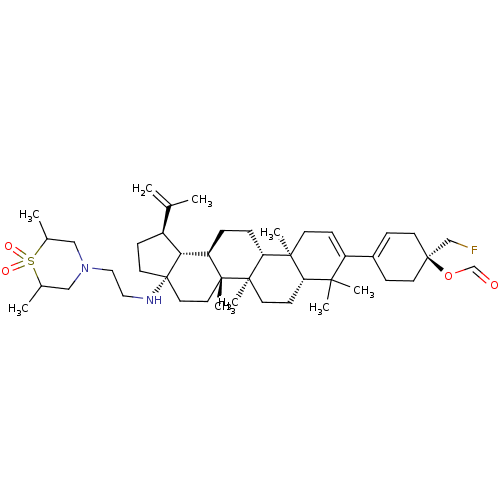
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sit, S; Chen, Y; Chen, J; Swidorski, J; Venables, BL; Sin, N; Meanwell, NA; Regueiro-Ren, A; Hartz, RA; Xu, L; Liu, Z Triterpenoids with HIV maturation inhibitory activity US Patent US10245275 Publication Date 4/2/2019
Sit, S; Chen, Y; Chen, J; Swidorski, J; Venables, BL; Sin, N; Meanwell, NA; Regueiro-Ren, A; Hartz, RA; Xu, L; Liu, Z Triterpenoids with HIV maturation inhibitory activity US Patent US10245275 Publication Date 4/2/2019