 Found 14188 hits with Last Name = 'liu' and Initial = 'z'
Found 14188 hits with Last Name = 'liu' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50557532

(CHEMBL4792421)Show SMILES COc1cc2C(=O)O\C(=C/c3ccc(O)c(c3)N3CCCCC3)c2cc1OC | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of Electrophorus electricus AChE using varying levels of acetylthiocholine iodide as substrate preincubated for 15 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116074
BindingDB Entry DOI: 10.7270/Q2HX1HBN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599186
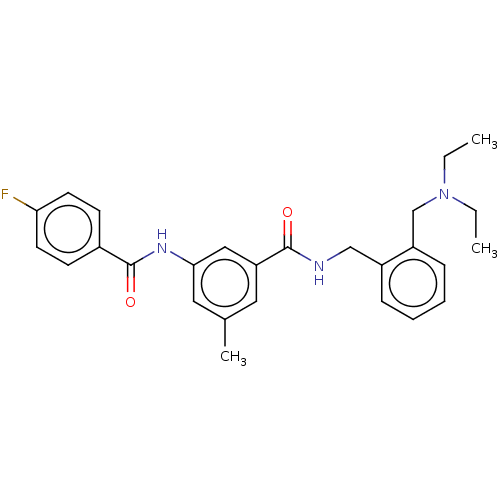
(CHEMBL5201089)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(C)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50091350
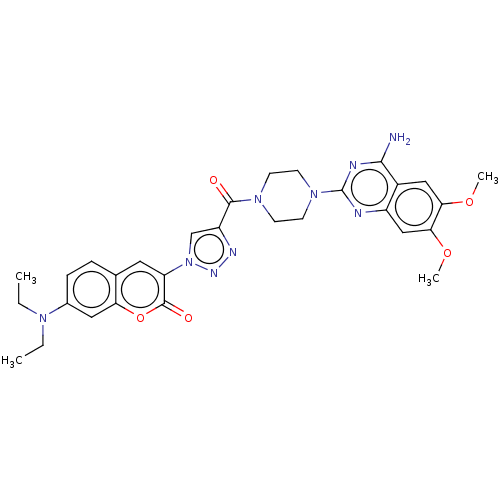
(CHEMBL3582270)Show SMILES CCN(CC)c1ccc2cc(-n3cc(nn3)C(=O)N3CCN(CC3)c3nc(N)c4cc(OC)c(OC)cc4n3)c(=O)oc2c1 Show InChI InChI=1S/C30H33N9O5/c1-5-36(6-2)19-8-7-18-13-23(29(41)44-24(18)14-19)39-17-22(34-35-39)28(40)37-9-11-38(12-10-37)30-32-21-16-26(43-4)25(42-3)15-20(21)27(31)33-30/h7-8,13-17H,5-6,9-12H2,1-4H3,(H2,31,32,33) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Prazosin from human alpha-1B adrenergic receptor transfected in CHO cell membranes after 2 hrs by microplate scintillation count... |
ACS Med Chem Lett 6: 502-6 (2015)
Article DOI: 10.1021/ml5004298
BindingDB Entry DOI: 10.7270/Q2VM4DZM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127387
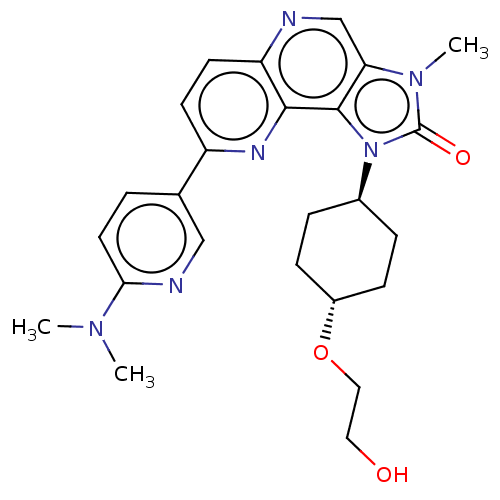
(US8791131, 149)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@@H](CC4)OCCO)c3c2n1 |r,wU:21.21,wD:24.28,(-7.32,-.2,;-5.98,.57,;-5.98,2.11,;-4.65,-.2,;-4.65,-1.74,;-3.32,-2.51,;-1.98,-1.74,;-1.98,-.2,;-3.32,.57,;-.65,-2.51,;-.65,-4.05,;.68,-4.82,;2.02,-4.05,;3.35,-4.82,;4.69,-4.05,;4.69,-2.51,;5.83,-1.48,;7.32,-1.87,;5.2,-.07,;5.97,1.26,;3.67,-.23,;2.58,.86,;1.1,.46,;.01,1.55,;.41,3.04,;1.89,3.44,;2.98,2.35,;-.68,4.13,;-2.17,3.73,;-3.26,4.82,;-4.75,4.42,;3.35,-1.74,;2.02,-2.51,;.68,-1.74,)| Show InChI InChI=1S/C25H30N6O3/c1-29(2)22-11-4-16(14-27-22)19-9-10-20-23(28-19)24-21(15-26-20)30(3)25(33)31(24)17-5-7-18(8-6-17)34-13-12-32/h4,9-11,14-15,17-18,32H,5-8,12-13H2,1-3H3/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.102 | -57.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50612016

(CHEMBL5172923) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127481
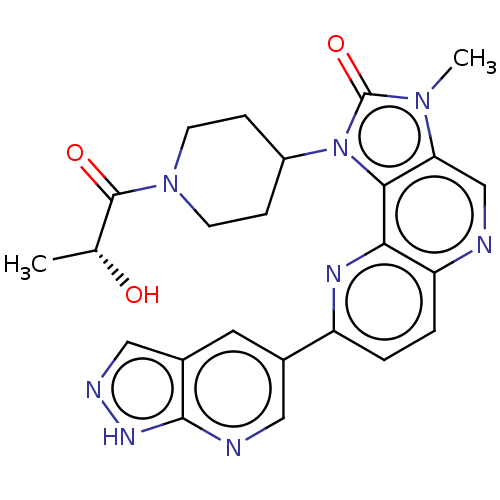
(US8791131, 258)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O |r| Show InChI InChI=1S/C24H24N8O3/c1-13(33)23(34)31-7-5-16(6-8-31)32-21-19(30(2)24(32)35)12-25-18-4-3-17(28-20(18)21)14-9-15-11-27-29-22(15)26-10-14/h3-4,9-13,16,33H,5-8H2,1-2H3,(H,26,27,29)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779
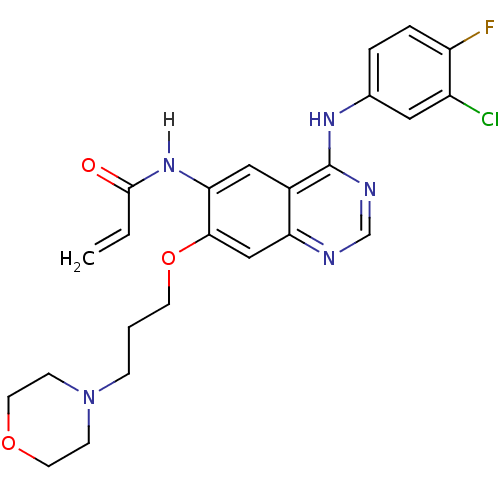
(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50428107
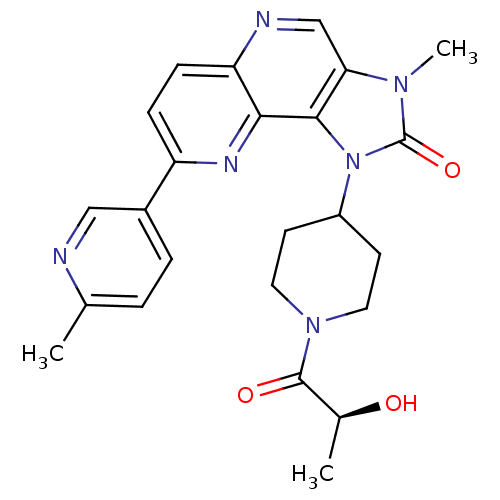
(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127483
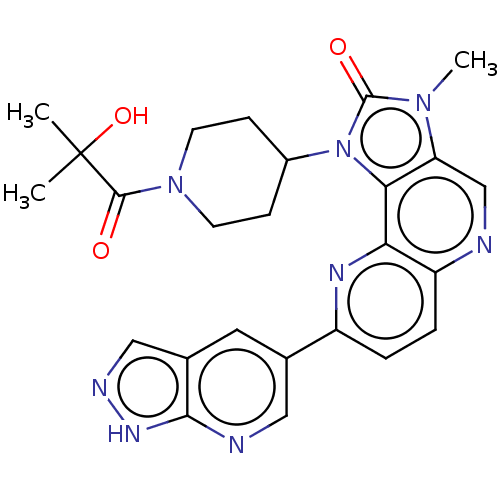
(US8791131, 261)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCN(CC2)C(=O)C(C)(C)O)c1=O)-c1cnc2[nH]ncc2c1 Show InChI InChI=1S/C25H26N8O3/c1-25(2,36)23(34)32-8-6-16(7-9-32)33-21-19(31(3)24(33)35)13-26-18-5-4-17(29-20(18)21)14-10-15-12-28-30-22(15)27-11-14/h4-5,10-13,16,36H,6-9H2,1-3H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.114 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127490
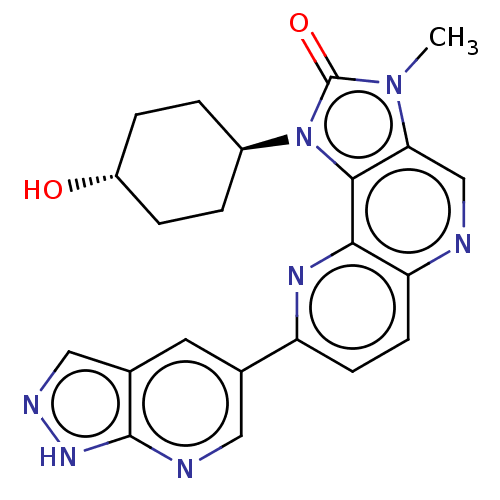
(US8791131, 268)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cnc2[nH]ncc2c1 |r,wU:13.14,wD:16.18,(7.27,-2.02,;5.78,-1.62,;4.64,-2.65,;4.64,-4.19,;3.31,-4.96,;1.97,-4.19,;.64,-4.96,;-.69,-4.19,;-.69,-2.65,;.64,-1.88,;1.97,-2.65,;3.31,-1.88,;3.63,-.37,;2.86,.96,;1.32,.96,;.55,2.29,;1.32,3.63,;.55,4.96,;2.86,3.63,;3.63,2.29,;5.16,-.21,;5.93,1.12,;-2.23,-2.65,;-2.23,-4.19,;-3.57,-4.96,;-4.9,-4.19,;-6.37,-4.67,;-7.27,-3.42,;-6.37,-2.17,;-4.9,-2.65,;-3.57,-1.88,)| Show InChI InChI=1S/C22H21N7O2/c1-28-18-11-23-17-7-6-16(12-8-13-10-25-27-21(13)24-9-12)26-19(17)20(18)29(22(28)31)14-2-4-15(30)5-3-14/h6-11,14-15,30H,2-5H2,1H3,(H,24,25,27)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.117 | -56.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598740
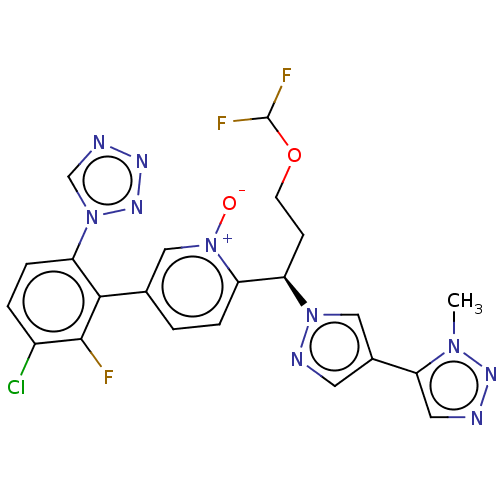
(CHEMBL5175227)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598738
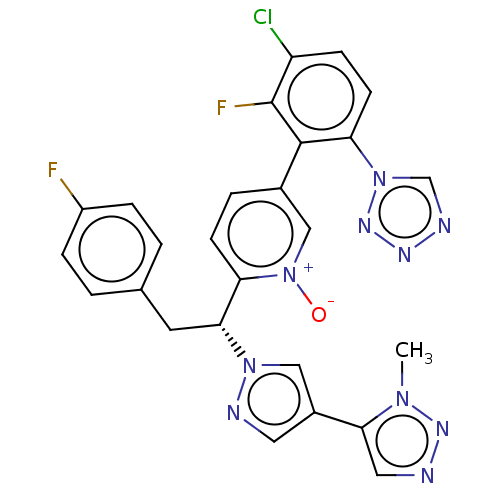
(CHEMBL5204065)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598739

(CHEMBL5188215)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127489
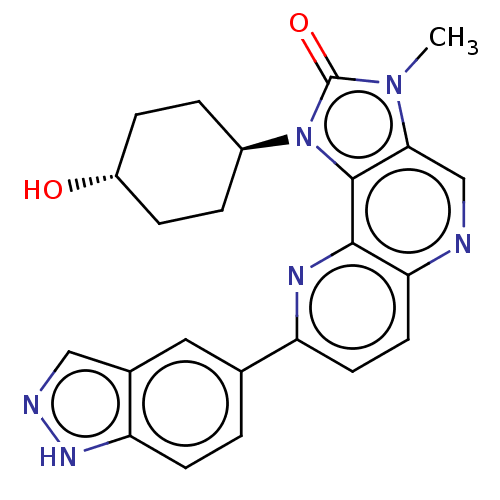
(US8791131, 267)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1ccc2[nH]ncc2c1 |r,wU:13.14,wD:16.18,(7.27,-2.02,;5.78,-1.62,;4.64,-2.65,;4.64,-4.19,;3.31,-4.96,;1.97,-4.19,;.64,-4.96,;-.69,-4.19,;-.69,-2.65,;.64,-1.88,;1.97,-2.65,;3.31,-1.88,;3.63,-.37,;2.86,.96,;1.32,.96,;.55,2.29,;1.32,3.63,;.55,4.96,;2.86,3.63,;3.63,2.29,;5.16,-.21,;5.93,1.12,;-2.23,-2.65,;-2.23,-4.19,;-3.57,-4.96,;-4.9,-4.19,;-6.37,-4.67,;-7.27,-3.42,;-6.37,-2.17,;-4.9,-2.65,;-3.57,-1.88,)| Show InChI InChI=1S/C23H22N6O2/c1-28-20-12-24-19-9-8-17(13-2-7-18-14(10-13)11-25-27-18)26-21(19)22(20)29(23(28)31)15-3-5-16(30)6-4-15/h2,7-12,15-16,30H,3-6H2,1H3,(H,25,27)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.145 | -56.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127482
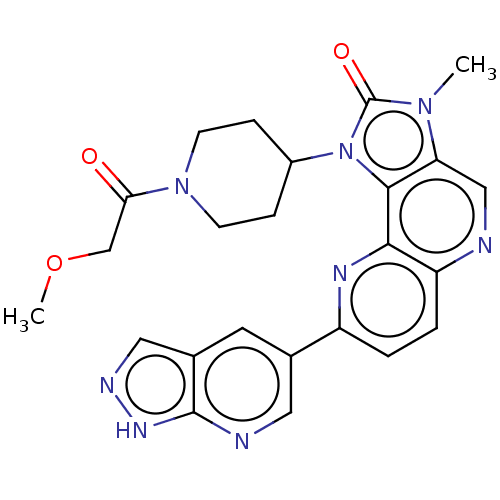
(US8791131, 260)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C24H24N8O3/c1-30-19-12-25-18-4-3-17(14-9-15-11-27-29-23(15)26-10-14)28-21(18)22(19)32(24(30)34)16-5-7-31(8-6-16)20(33)13-35-2/h3-4,9-12,16H,5-8,13H2,1-2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.167 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504229

(N-[2-[(2R)-2-Fluoro-3-hydroxy- 3-methyl-butyl]-6-i...)Show SMILES CC(C)Oc1cc2C(=O)N(C[C@@H](F)C(C)(C)O)Cc2cc1NC(=O)c1cnn2cccnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM60828
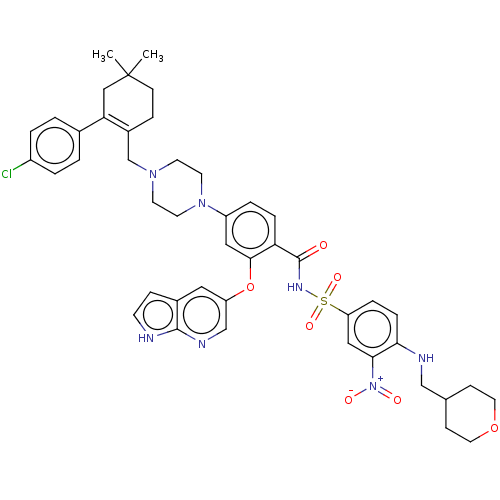
(ABT-199 | BDBM189459 | US10213433, Compound 5 | US...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2P84H1R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428109
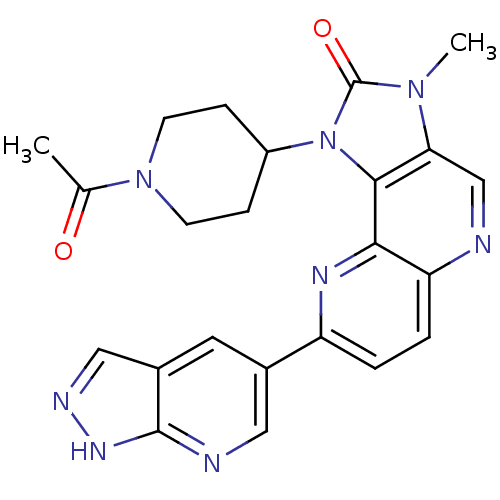
(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.191 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428109

(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001019
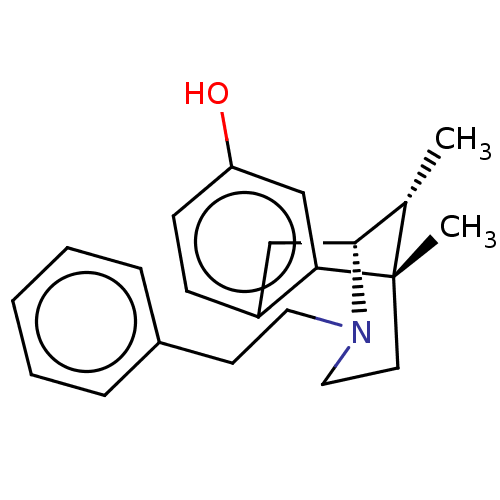
(6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CCc1ccccc1 |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C22H27NO/c1-16-21-14-18-8-9-19(24)15-20(18)22(16,2)11-13-23(21)12-10-17-6-4-3-5-7-17/h3-9,15-16,21,24H,10-14H2,1-2H3/t16-,21+,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598737
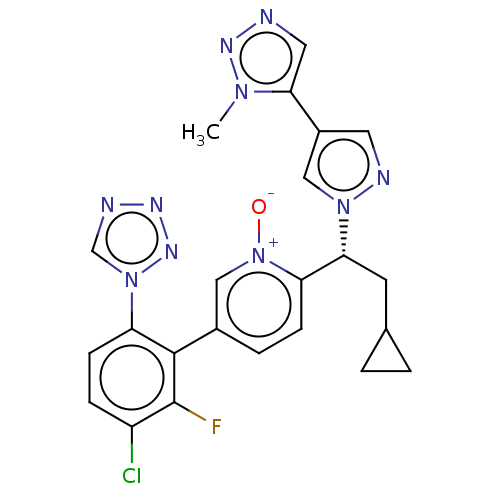
(CHEMBL5205631)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343977
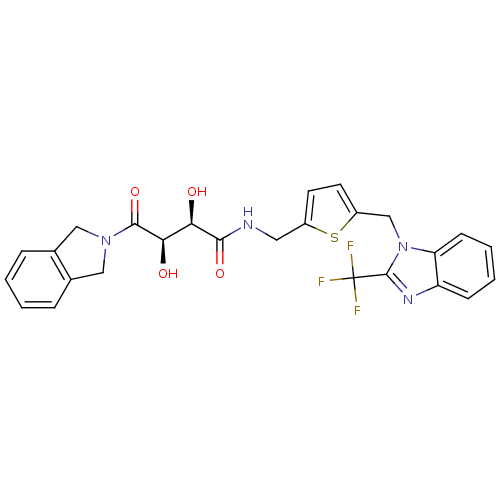
((2R,3R)-2,3-dihydroxy-4-(isoindolin-2-yl)-4-oxo-N-...)Show SMILES O[C@H]([C@@H](O)C(=O)N1Cc2ccccc2C1)C(=O)NCc1ccc(Cn2c(nc3ccccc23)C(F)(F)F)s1 |r| Show InChI InChI=1S/C26H23F3N4O4S/c27-26(28,29)25-31-19-7-3-4-8-20(19)33(25)14-18-10-9-17(38-18)11-30-23(36)21(34)22(35)24(37)32-12-15-5-1-2-6-16(15)13-32/h1-10,21-22,34-35H,11-14H2,(H,30,36)/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127403
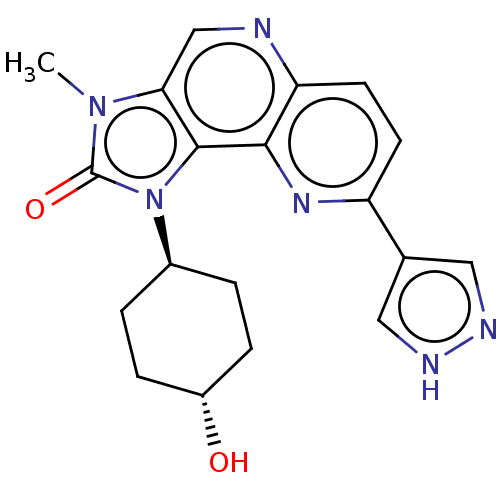
(US8791131, 168)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cn[nH]c1 |r,wU:13.14,wD:16.18,(5.84,-1.53,;4.35,-1.13,;3.2,-2.16,;3.2,-3.7,;1.87,-4.47,;.54,-3.7,;-.8,-4.47,;-2.13,-3.7,;-2.13,-2.16,;-.8,-1.39,;.54,-2.16,;1.87,-1.39,;2.19,.12,;1.1,1.2,;-.39,.81,;-1.48,1.89,;-1.08,3.38,;-2.17,4.47,;.41,3.78,;1.5,2.69,;3.72,.28,;4.49,1.61,;-3.47,-1.39,;-4.93,-1.87,;-5.84,-.62,;-4.93,.62,;-3.47,.15,)| Show InChI InChI=1S/C19H20N6O2/c1-24-16-10-20-15-7-6-14(11-8-21-22-9-11)23-17(15)18(16)25(19(24)27)12-2-4-13(26)5-3-12/h6-10,12-13,26H,2-5H2,1H3,(H,21,22)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.204 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129823
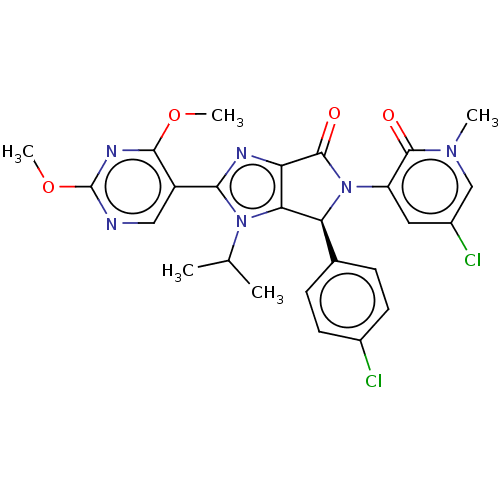
(US8815926, 102)Show SMILES COc1ncc(-c2nc3C(=O)N([C@H](c3n2C(C)C)c2ccc(Cl)cc2)c2cc(Cl)cn(C)c2=O)c(OC)n1 |r| Show InChI InChI=1S/C26H24Cl2N6O4/c1-13(2)33-21-19(30-22(33)17-11-29-26(38-5)31-23(17)37-4)25(36)34(18-10-16(28)12-32(3)24(18)35)20(21)14-6-8-15(27)9-7-14/h6-13,20H,1-5H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Institute for Translational Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) |
Eur J Med Chem 159: 1-9 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.044
BindingDB Entry DOI: 10.7270/Q2SB48FV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598741

(CHEMBL5204894)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127481

(US8791131, 258)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O |r| Show InChI InChI=1S/C24H24N8O3/c1-13(33)23(34)31-7-5-16(6-8-31)32-21-19(30(2)24(32)35)12-25-18-4-3-17(28-20(18)21)14-9-15-11-27-29-22(15)26-10-14/h3-4,9-13,16,33H,5-8H2,1-2H3,(H,26,27,29)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.241 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428109

(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.243 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428109

(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598725

(CHEMBL5185397)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127468
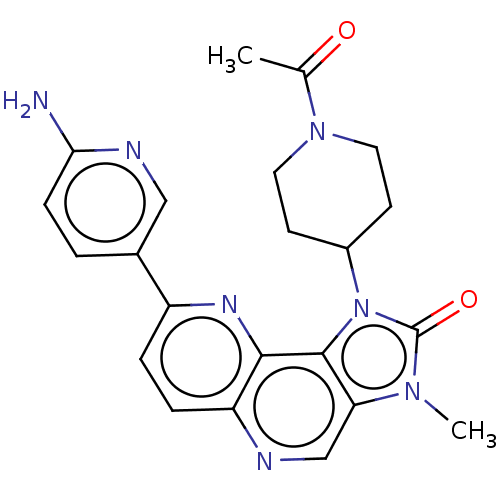
(US8791131, 242)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(N)nc2)n(C)c1=O Show InChI InChI=1S/C22H23N7O2/c1-13(30)28-9-7-15(8-10-28)29-21-18(27(2)22(29)31)12-24-17-5-4-16(26-20(17)21)14-3-6-19(23)25-11-14/h3-6,11-12,15H,7-10H2,1-2H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.252 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598743
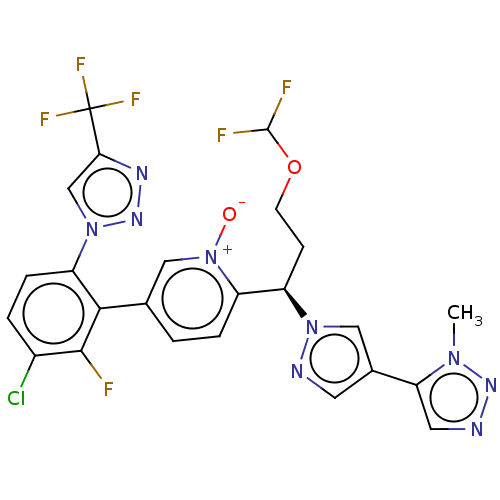
(CHEMBL5178223)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504219
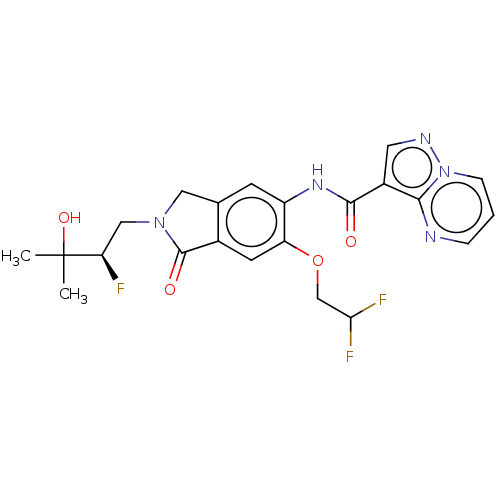
((R)-N-(6-(2,2-Difluoroethoxy)- 2-(2-fluoro-3-hydro...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(OCC(F)F)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127435
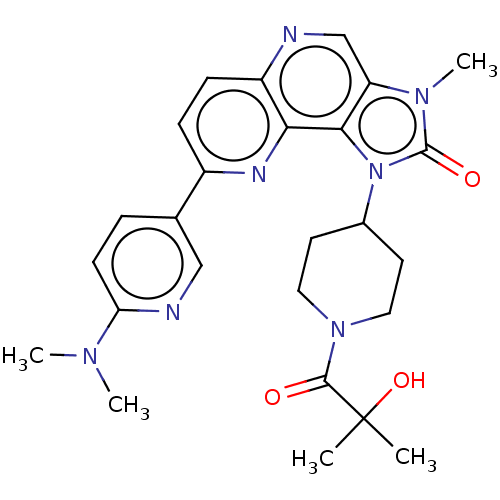
(US8791131, 209)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)C(C)(C)O)c3c2n1 Show InChI InChI=1S/C26H31N7O3/c1-26(2,36)24(34)32-12-10-17(11-13-32)33-23-20(31(5)25(33)35)15-27-19-8-7-18(29-22(19)23)16-6-9-21(28-14-16)30(3)4/h6-9,14-15,17,36H,10-13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.264 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127455
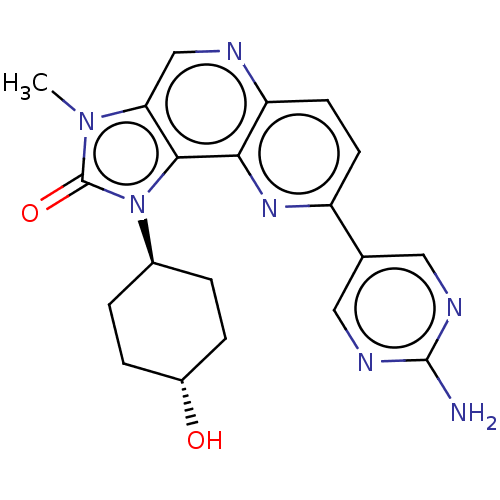
(US8791131, 229)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cnc(N)nc1 |r,wU:13.14,wD:16.18,(6.65,-1.53,;5.16,-1.13,;4.02,-2.16,;4.02,-3.7,;2.69,-4.47,;1.35,-3.7,;.02,-4.47,;-1.32,-3.7,;-1.32,-2.16,;.02,-1.39,;1.35,-2.16,;2.69,-1.39,;3.01,.12,;1.92,1.2,;.43,.81,;-.66,1.89,;-.26,3.38,;-1.35,4.47,;1.23,3.78,;2.31,2.69,;4.54,.28,;5.31,1.61,;-2.65,-1.39,;-2.65,.15,;-3.98,.92,;-5.32,.15,;-6.65,.92,;-5.32,-1.39,;-3.98,-2.16,)| Show InChI InChI=1S/C20H21N7O2/c1-26-16-10-22-15-7-6-14(11-8-23-19(21)24-9-11)25-17(15)18(16)27(20(26)29)12-2-4-13(28)5-3-12/h6-10,12-13,28H,2-5H2,1H3,(H2,21,23,24)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.273 | -54.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504215

(N-[6-(3-Fluorocyclobutoxy)-2- [(2R)-2-fluoro-3-hyd...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(O[C@@H]3C[C@H](F)C3)cc2C1=O |r,wU:26.27,28.30,wD:4.4,(-7.2,.34,;-7.24,1.88,;-8.73,1.48,;-8.01,3.22,;-5.7,1.88,;-4.93,3.22,;-4.93,.55,;-3.39,.55,;-2.49,1.79,;-1.02,1.32,;.31,2.09,;1.64,1.32,;2.98,2.09,;2.98,3.63,;1.64,4.4,;4.31,4.4,;4.47,5.93,;5.98,6.25,;6.75,4.92,;8.25,4.6,;8.73,3.13,;7.7,1.99,;6.19,2.31,;5.72,3.77,;1.64,-.22,;2.98,-.99,;2.98,-2.53,;1.89,-3.62,;2.98,-4.71,;2.98,-6.25,;4.07,-3.62,;.31,-.99,;-1.02,-.22,;-2.49,-.7,;-2.96,-2.16,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127431
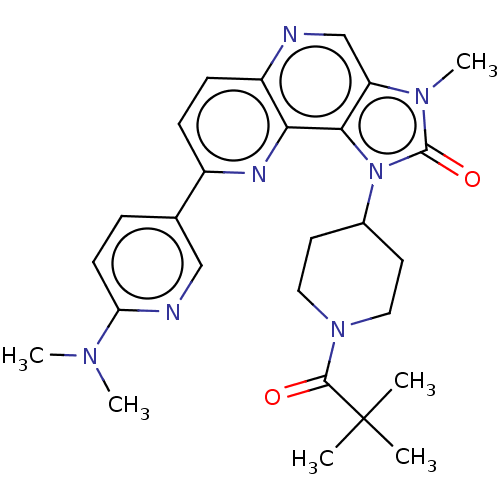
(US8791131, 205)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)C(C)(C)C)c3c2n1 Show InChI InChI=1S/C27H33N7O2/c1-27(2,3)25(35)33-13-11-18(12-14-33)34-24-21(32(6)26(34)36)16-28-20-9-8-19(30-23(20)24)17-7-10-22(29-15-17)31(4)5/h7-10,15-16,18H,11-14H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.281 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
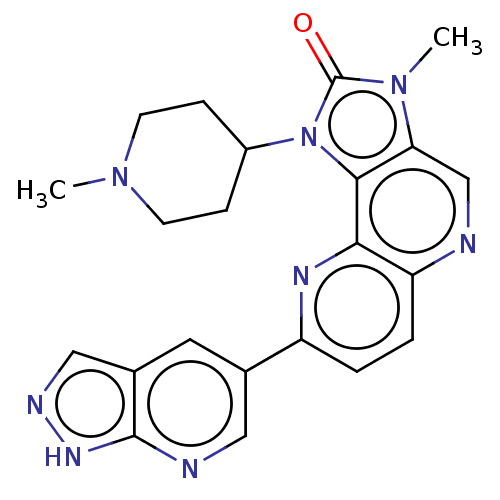
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127485
(US8791131, 263)Show SMILES CN1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C22H22N8O/c1-28-7-5-15(6-8-28)30-20-18(29(2)22(30)31)12-23-17-4-3-16(26-19(17)20)13-9-14-11-25-27-21(14)24-10-13/h3-4,9-12,15H,5-8H2,1-2H3,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.282 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
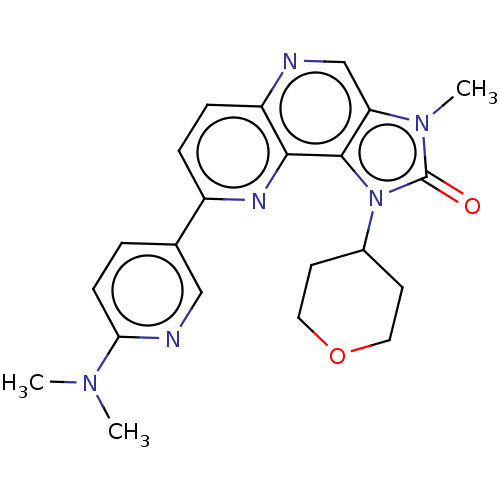
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127430
(US8791131, 204)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2n1 Show InChI InChI=1S/C22H24N6O2/c1-26(2)19-7-4-14(12-24-19)16-5-6-17-20(25-16)21-18(13-23-17)27(3)22(29)28(21)15-8-10-30-11-9-15/h4-7,12-13,15H,8-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.298 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127468

(US8791131, 242)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(N)nc2)n(C)c1=O Show InChI InChI=1S/C22H23N7O2/c1-13(30)28-9-7-15(8-10-28)29-21-18(27(2)22(29)31)12-24-17-5-4-16(26-20(17)21)14-3-6-19(23)25-11-14/h3-6,11-12,15H,7-10H2,1-2H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.299 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598745
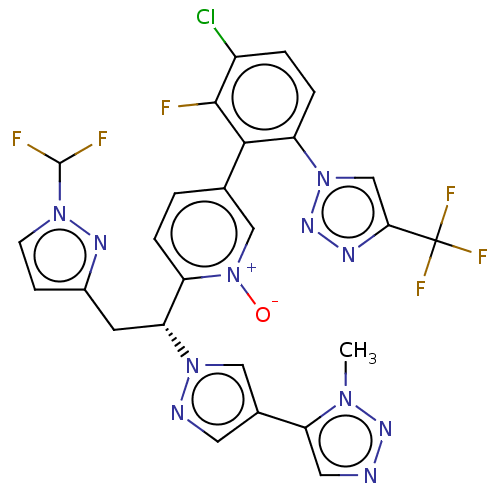
(CHEMBL5198823)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccn(n1)C(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50091350

(CHEMBL3582270)Show SMILES CCN(CC)c1ccc2cc(-n3cc(nn3)C(=O)N3CCN(CC3)c3nc(N)c4cc(OC)c(OC)cc4n3)c(=O)oc2c1 Show InChI InChI=1S/C30H33N9O5/c1-5-36(6-2)19-8-7-18-13-23(29(41)44-24(18)14-19)39-17-22(34-35-39)28(40)37-9-11-38(12-10-37)30-32-21-16-26(43-4)25(42-3)15-20(21)27(31)33-30/h7-8,13-17H,5-6,9-12H2,1-4H3,(H2,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Prazosin from human alpha-1A adrenergic receptor transfected in CHO cell membranes after 2 hrs by microplate scintillation count... |
ACS Med Chem Lett 6: 502-6 (2015)
Article DOI: 10.1021/ml5004298
BindingDB Entry DOI: 10.7270/Q2VM4DZM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50334346
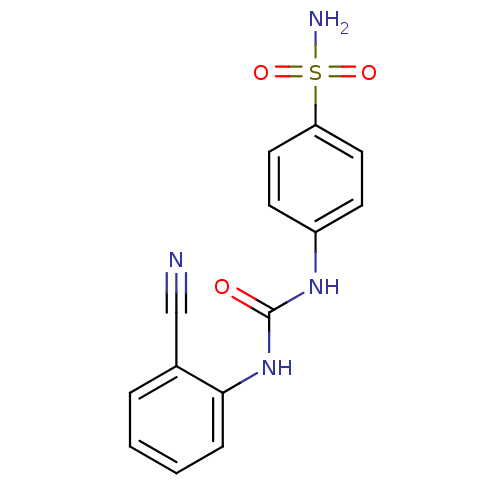
(4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...)Show InChI InChI=1S/C14H12N4O3S/c15-9-10-3-1-2-4-13(10)18-14(19)17-11-5-7-12(8-6-11)22(16,20)21/h1-8H,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency
| Assay Description
The inhibition constants (Ki) of FEC to four human CA isoenzymes I, II, IX and XII were determined by CA catalyzed CO2 hydration assays following pre... |
J Enzyme Inhib Med Chem 29: 249-55 (2014)
Article DOI: 10.3109/14756366.2013.773994
BindingDB Entry DOI: 10.7270/Q2VM4B6X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50582411
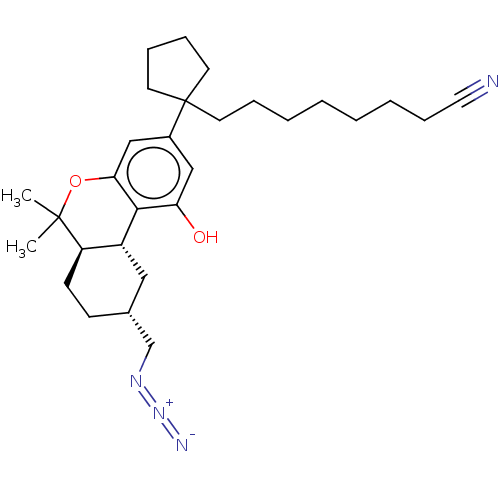
(CHEMBL5084325)Show SMILES [H][C@@]12C[C@H](CN=[N+]=[N-])CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCCCC#N)CCCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02053
BindingDB Entry DOI: 10.7270/Q2TB1BSB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50582411

(CHEMBL5084325)Show SMILES [H][C@@]12C[C@H](CN=[N+]=[N-])CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C1(CCCCCCCC#N)CCCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02053
BindingDB Entry DOI: 10.7270/Q2TB1BSB |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598734
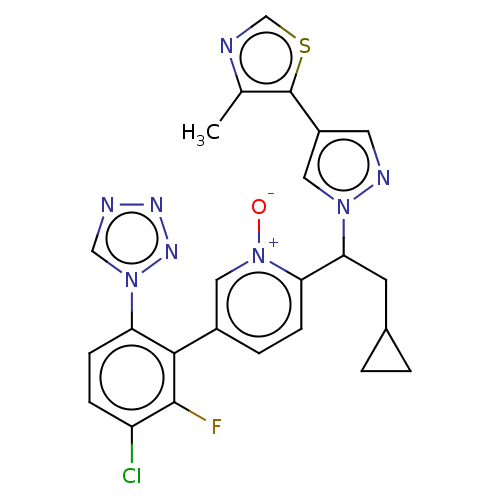
(CHEMBL5197480)Show SMILES Cc1ncsc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127411
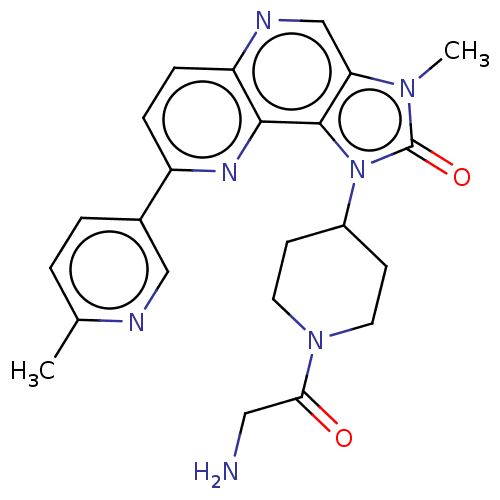
(US8791131, 180)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CN)c3c2n1 Show InChI InChI=1S/C23H25N7O2/c1-14-3-4-15(12-25-14)17-5-6-18-21(27-17)22-19(13-26-18)28(2)23(32)30(22)16-7-9-29(10-8-16)20(31)11-24/h3-6,12-13,16H,7-11,24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.320 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data