| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-arrestin-2 |
|---|
| Ligand | BDBM50029150 |
|---|
| Substrate/Competitor | n/a |
|---|
| Ki | 3235.93±n/a nM |
|---|
| Comments | PDSP_976 |
|---|
| Citation |  Wong, DT; Threlkeld, PG; Robertson, DW Affinities of fluoxetine, its enantiomers, and other inhibitors of serotonin uptake for subtypes of serotonin receptors. Neuropsychopharmacology5:43-7 (1991) [PubMed] Wong, DT; Threlkeld, PG; Robertson, DW Affinities of fluoxetine, its enantiomers, and other inhibitors of serotonin uptake for subtypes of serotonin receptors. Neuropsychopharmacology5:43-7 (1991) [PubMed] |
|---|
| More Info.: | Get all data from this article |
|---|
| |
| Beta-arrestin-2 |
|---|
| Name: | Beta-arrestin-2 |
|---|
| Synonyms: | 5-HT2C | ARRB2 | ARRB2_BOVIN | Beta-arrestin-2 | HTR2C |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 47232.00 |
|---|
| Organism: | Beef |
|---|
| Description: | 5-HT2C HTR2C Beef::P32120 |
|---|
| Residue: | 420 |
|---|
| Sequence: | MGEKPGTRVFKKSSPNCKLTVYLGKRDFVDHLDKVDPVDGVVLVDPDYLKDRKVFVTLTC
AFRYGREDLDVLGLSFRKDLFIANYQAFPPTPNPPRPPTRLQERLLRKLGQHAHPFFFTI
PQNLPCSVTLQPGPEDTGKACGVDFEIRAFCAKSLEEKSHKRNSVRLVIRKVQFAPEKPG
PQPSAETTRHFLMSDRSLHLEASLDKELYYHGEPLNVNVHVTNNSTKTVKKIKVSVRQYA
DICLFSTAQYKCPVAQVEQDDQVSPSSTFCKVYTITPLLSNNREKRGLALDGKLKHEDTN
LASSTIVKEGANKEVLGILVSYRVKVKLVVSRGGDVSVELPFVLMHPKPHDHIALPRPQS
AATHPPTLLPSAVPETDAPVDTNLIEFETNYATDDDIVFEDFARLRLKGLKDEDYDDQFC
|
|
|
|---|
| BDBM50029150 |
|---|
| n/a |
|---|
| Name | BDBM50029150 |
|---|
| Synonyms: | 3-(2-Piperidin-4-yl-ethyl)-1H-indole | CHEMBL276520 | Indalpine |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H20N2 |
|---|
| Mol. Mass. | 228.3327 |
|---|
| SMILES | C(Cc1c[nH]c2ccccc12)C1CCNCC1 |
|---|
| Structure | 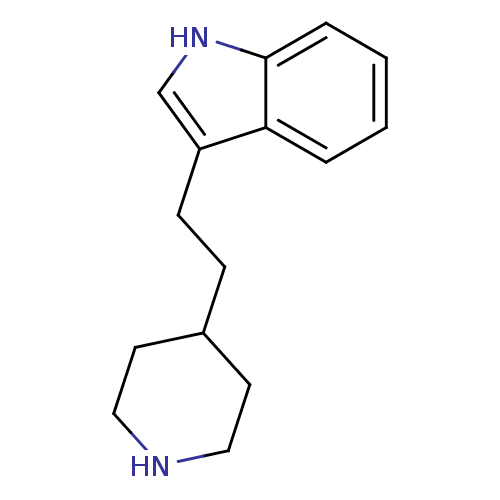
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wong, DT; Threlkeld, PG; Robertson, DW Affinities of fluoxetine, its enantiomers, and other inhibitors of serotonin uptake for subtypes of serotonin receptors. Neuropsychopharmacology5:43-7 (1991) [PubMed]
Wong, DT; Threlkeld, PG; Robertson, DW Affinities of fluoxetine, its enantiomers, and other inhibitors of serotonin uptake for subtypes of serotonin receptors. Neuropsychopharmacology5:43-7 (1991) [PubMed]