| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor tyrosine-protein kinase erbB-4 |
|---|
| Ligand | BDBM50269555 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1706723 (CHEMBL4057956) |
|---|
| IC50 | 76±n/a nM |
|---|
| Citation |  Boga, SB; Alhassan, AB; Liu, J; Guiadeen, D; Krikorian, A; Gao, X; Wang, J; Yu, Y; Anand, R; Liu, S; Yang, C; Wu, H; Cai, J; Zhu, H; Desai, J; Maloney, K; Gao, YD; Fischmann, TO; Presland, J; Mansueto, M; Xu, Z; Leccese, E; Knemeyer, I; Garlisi, CG; Bays, N; Stivers, P; Brandish, PE; Hicks, A; Cooper, A; Kim, RM; Kozlowski, JA Discovery of 3-morpholino-imidazole[1,5-a]pyrazine BTK inhibitors for rheumatoid arthritis. Bioorg Med Chem Lett27:3939-3943 (2017) [PubMed] Article Boga, SB; Alhassan, AB; Liu, J; Guiadeen, D; Krikorian, A; Gao, X; Wang, J; Yu, Y; Anand, R; Liu, S; Yang, C; Wu, H; Cai, J; Zhu, H; Desai, J; Maloney, K; Gao, YD; Fischmann, TO; Presland, J; Mansueto, M; Xu, Z; Leccese, E; Knemeyer, I; Garlisi, CG; Bays, N; Stivers, P; Brandish, PE; Hicks, A; Cooper, A; Kim, RM; Kozlowski, JA Discovery of 3-morpholino-imidazole[1,5-a]pyrazine BTK inhibitors for rheumatoid arthritis. Bioorg Med Chem Lett27:3939-3943 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor tyrosine-protein kinase erbB-4 |
|---|
| Name: | Receptor tyrosine-protein kinase erbB-4 |
|---|
| Synonyms: | ERBB4 | ERBB4_HUMAN | Epidermal growth factor receptor | Epidermal growth factor receptor 4 (ERBB4) | ErbB-4 (HER4) Tyrosine Kinase | HER4 | Proto-oncogene c-ErbB-4 | Proto-oncogene-like protein c-ErbB-4 | Receptor protein-tyrosine kinase erbB-4 | Tyrosine kinase-type cell surface receptor HER4 | Tyrosine kinase-type cell surface receptor HER4 (HER4) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 146803.56 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q15303 |
|---|
| Residue: | 1308 |
|---|
| Sequence: | MKPATGLWVWVSLLVAAGTVQPSDSQSVCAGTENKLSSLSDLEQQYRALRKYYENCEVVM
GNLEITSIEHNRDLSFLRSVREVTGYVLVALNQFRYLPLENLRIIRGTKLYEDRYALAIF
LNYRKDGNFGLQELGLKNLTEILNGGVYVDQNKFLCYADTIHWQDIVRNPWPSNLTLVST
NGSSGCGRCHKSCTGRCWGPTENHCQTLTRTVCAEQCDGRCYGPYVSDCCHRECAGGCSG
PKDTDCFACMNFNDSGACVTQCPQTFVYNPTTFQLEHNFNAKYTYGAFCVKKCPHNFVVD
SSSCVRACPSSKMEVEENGIKMCKPCTDICPKACDGIGTGSLMSAQTVDSSNIDKFINCT
KINGNLIFLVTGIHGDPYNAIEAIDPEKLNVFRTVREITGFLNIQSWPPNMTDFSVFSNL
VTIGGRVLYSGLSLLILKQQGITSLQFQSLKEISAGNIYITDNSNLCYYHTINWTTLFST
INQRIVIRDNRKAENCTAEGMVCNHLCSSDGCWGPGPDQCLSCRRFSRGRICIESCNLYD
GEFREFENGSICVECDPQCEKMEDGLLTCHGPGPDNCTKCSHFKDGPNCVEKCPDGLQGA
NSFIFKYADPDRECHPCHPNCTQGCNGPTSHDCIYYPWTGHSTLPQHARTPLIAAGVIGG
LFILVIVGLTFAVYVRRKSIKKKRALRRFLETELVEPLTPSGTAPNQAQLRILKETELKR
VKVLGSGAFGTVYKGIWVPEGETVKIPVAIKILNETTGPKANVEFMDEALIMASMDHPHL
VRLLGVCLSPTIQLVTQLMPHGCLLEYVHEHKDNIGSQLLLNWCVQIAKGMMYLEERRLV
HRDLAARNVLVKSPNHVKITDFGLARLLEGDEKEYNADGGKMPIKWMALECIHYRKFTHQ
SDVWSYGVTIWELMTFGGKPYDGIPTREIPDLLEKGERLPQPPICTIDVYMVMVKCWMID
ADSRPKFKELAAEFSRMARDPQRYLVIQGDDRMKLPSPNDSKFFQNLLDEEDLEDMMDAE
EYLVPQAFNIPPPIYTSRARIDSNRSEIGHSPPPAYTPMSGNQFVYRDGGFAAEQGVSVP
YRAPTSTIPEAPVAQGATAEIFDDSCCNGTLRKPVAPHVQEDSSTQRYSADPTVFAPERS
PRGELDEEGYMTPMRDKPKQEYLNPVEENPFVSRRKNGDLQALDNPEYHNASNGPPKAED
EYVNEPLYLNTFANTLGKAEYLKNNILSMPEKAKKAFDNPDYWNHSLPPRSTLQHPDYLQ
EYSTKYFYKQNGRIRPIVAENPEYLSEFSLKPGTVLPPPPYRHRNTVV
|
|
|
|---|
| BDBM50269555 |
|---|
| n/a |
|---|
| Name | BDBM50269555 |
|---|
| Synonyms: | CHEMBL4060757 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H27F4N7O3 |
|---|
| Mol. Mass. | 585.5527 |
|---|
| SMILES | Nc1nccn2c(nc(-c3ccc(cc3F)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@H]1CN(CCO1)C1CCOCC1 |r| |
|---|
| Structure | 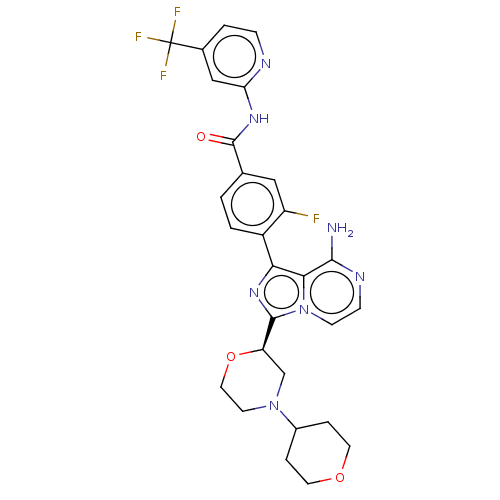
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Boga, SB; Alhassan, AB; Liu, J; Guiadeen, D; Krikorian, A; Gao, X; Wang, J; Yu, Y; Anand, R; Liu, S; Yang, C; Wu, H; Cai, J; Zhu, H; Desai, J; Maloney, K; Gao, YD; Fischmann, TO; Presland, J; Mansueto, M; Xu, Z; Leccese, E; Knemeyer, I; Garlisi, CG; Bays, N; Stivers, P; Brandish, PE; Hicks, A; Cooper, A; Kim, RM; Kozlowski, JA Discovery of 3-morpholino-imidazole[1,5-a]pyrazine BTK inhibitors for rheumatoid arthritis. Bioorg Med Chem Lett27:3939-3943 (2017) [PubMed] Article
Boga, SB; Alhassan, AB; Liu, J; Guiadeen, D; Krikorian, A; Gao, X; Wang, J; Yu, Y; Anand, R; Liu, S; Yang, C; Wu, H; Cai, J; Zhu, H; Desai, J; Maloney, K; Gao, YD; Fischmann, TO; Presland, J; Mansueto, M; Xu, Z; Leccese, E; Knemeyer, I; Garlisi, CG; Bays, N; Stivers, P; Brandish, PE; Hicks, A; Cooper, A; Kim, RM; Kozlowski, JA Discovery of 3-morpholino-imidazole[1,5-a]pyrazine BTK inhibitors for rheumatoid arthritis. Bioorg Med Chem Lett27:3939-3943 (2017) [PubMed] Article