| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Carboxypeptidase B2 |
|---|
| Ligand | BDBM50275212 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1714513 (CHEMBL4124562) |
|---|
| IC50 | 1.5±n/a nM |
|---|
| Citation |  Itoh, T; Yoshimoto, N; Hirano, Y; Yamamoto, K Structural basis for the selective inhibition of activated thrombin-activatable fibrinolysis inhibitor (TAFIa) by a selenium-containing inhibitor with chloro-aminopyridine as a basic group. Bioorg Med Chem Lett28:2256-2260 (2018) [PubMed] Article Itoh, T; Yoshimoto, N; Hirano, Y; Yamamoto, K Structural basis for the selective inhibition of activated thrombin-activatable fibrinolysis inhibitor (TAFIa) by a selenium-containing inhibitor with chloro-aminopyridine as a basic group. Bioorg Med Chem Lett28:2256-2260 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Carboxypeptidase B2 |
|---|
| Name: | Carboxypeptidase B2 |
|---|
| Synonyms: | CBPB2_HUMAN | CPB2 | CPU | Carboxypeptidase B2 | Carboxypeptidase B2 isoform A | Carboxypeptidase U | Plasma carboxypeptidase B | TAFI | Thrombin-activable fibrinolysis inhibitor | pCPB |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48432.74 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q96IY4 |
|---|
| Residue: | 423 |
|---|
| Sequence: | MKLCSLAVLVPIVLFCEQHVFAFQSGQVLAALPRTSRQVQVLQNLTTTYEIVLWQPVTAD
LIVKKKQVHFFVNASDVDNVKAHLNVSGIPCSVLLADVEDLIQQQISNDTVSPRASASYY
EQYHSLNEIYSWIEFITERHPDMLTKIHIGSSFEKYPLYVLKVSGKEQAAKNAIWIDCGI
HAREWISPAFCLWFIGHITQFYGIIGQYTNLLRLVDFYVMPVVNVDGYDYSWKKNRMWRK
NRSFYANNHCIGTDLNRNFASKHWCEEGASSSSCSETYCGLYPESEPEVKAVASFLRRNI
NQIKAYISMHSYSQHIVFPYSYTRSKSKDHEELSLVASEAVRAIEKISKNTRYTHGHGSE
TLYLAPGGGDDWIYDLGIKYSFTIELRDTGTYGFLLPERYIKPTCREAFAAVSKIAWHVI
RNV
|
|
|
|---|
| BDBM50275212 |
|---|
| n/a |
|---|
| Name | BDBM50275212 |
|---|
| Synonyms: | CHEMBL4127473 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C12H15ClN2O3Se |
|---|
| Mol. Mass. | 349.67 |
|---|
| SMILES | [#6]-[#6]-[#6](=O)[Se;v2][#6]-[#6](-[#6]-c1cnc(-[#7])c(Cl)c1)-[#6](-[#8])=O |
|---|
| Structure | 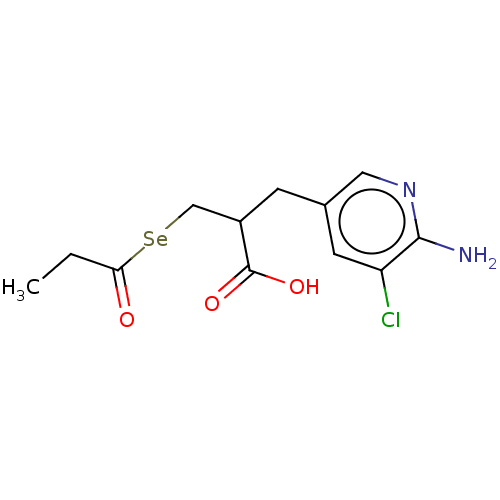
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Itoh, T; Yoshimoto, N; Hirano, Y; Yamamoto, K Structural basis for the selective inhibition of activated thrombin-activatable fibrinolysis inhibitor (TAFIa) by a selenium-containing inhibitor with chloro-aminopyridine as a basic group. Bioorg Med Chem Lett28:2256-2260 (2018) [PubMed] Article
Itoh, T; Yoshimoto, N; Hirano, Y; Yamamoto, K Structural basis for the selective inhibition of activated thrombin-activatable fibrinolysis inhibitor (TAFIa) by a selenium-containing inhibitor with chloro-aminopyridine as a basic group. Bioorg Med Chem Lett28:2256-2260 (2018) [PubMed] Article