| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sphingomyelin phosphodiesterase |
|---|
| Ligand | BDBM55861 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1737276 (CHEMBL4153026) |
|---|
| IC50 | 31623±n/a nM |
|---|
| Citation |  Yang, K; Nong, K; Gu, Q; Dong, J; Wang, J Discovery of N-hydroxy-3-alkoxybenzamides as direct acid sphingomyelinase inhibitors using a ligand-based pharmacophore model. Eur J Med Chem151:389-400 (2018) [PubMed] Article Yang, K; Nong, K; Gu, Q; Dong, J; Wang, J Discovery of N-hydroxy-3-alkoxybenzamides as direct acid sphingomyelinase inhibitors using a ligand-based pharmacophore model. Eur J Med Chem151:389-400 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sphingomyelin phosphodiesterase |
|---|
| Name: | Sphingomyelin phosphodiesterase |
|---|
| Synonyms: | 3.1.4.12 | ASM | ASM_HUMAN | Acid sphingomyelinase | SMPD1 | Sphingomyelin phosphodiesterase | aSMase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 69945.03 |
|---|
| Organism: | Homo sapiens |
|---|
| Description: | ChEMBL_11570 |
|---|
| Residue: | 631 |
|---|
| Sequence: | MPRYGASLRQSCPRSGREQGQDGTAGAPGLLWMGLVLALALALALALALSDSRVLWAPAE
AHPLSPQGHPARLHRIVPRLRDVFGWGNLTCPICKGLFTAINLGLKKEPNVARVGSVAIK
LCNLLKIAPPAVCQSIVHLFEDDMVEVWRRSVLSPSEACGLLLGSTCGHWDIFSSWNISL
PTVPKPPPKPPSPPAPGAPVSRILFLTDLHWDHDYLEGTDPDCADPLCCRRGSGLPPASR
PGAGYWGEYSKCDLPLRTLESLLSGLGPAGPFDMVYWTGDIPAHDVWHQTRQDQLRALTT
VTALVRKFLGPVPVYPAVGNHESTPVNSFPPPFIEGNHSSRWLYEAMAKAWEPWLPAEAL
RTLRIGGFYALSPYPGLRLISLNMNFCSRENFWLLINSTDPAGQLQWLVGELQAAEDRGD
KVHIIGHIPPGHCLKSWSWNYYRIVARYENTLAAQFFGHTHVDEFEVFYDEETLSRPLAV
AFLAPSATTYIGLNPGYRVYQIDGNYSGSSHVVLDHETYILNLTQANIPGAIPHWQLLYR
ARETYGLPNTLPTAWHNLVYRMRGDMQLFQTFWFLYHKGHPPSEPCGTPCRLATLCAQLS
ARADSPALCRHLMPDGSLPEAQSLWPRPLFC
|
|
|
|---|
| BDBM55861 |
|---|
| n/a |
|---|
| Name | BDBM55861 |
|---|
| Synonyms: | 5-Acetoxy-6-bromo-2-carbamimidoylsulfanylmethyl-1-methyl-1H-indole-3-carboxylic acid octyl ester | 5-acetoxy-2-[(amidinothio)methyl]-6-bromo-1-methyl-indole-3-carboxylic acid octyl ester;hydrobromide | 5-acetyloxy-6-bromo-2-[(carbamimidoylthio)methyl]-1-methyl-3-indolecarboxylic acid octyl ester;hydrobromide | MLS001029434 | SMR000425551 | cid_16681981 | octyl 5-acetyloxy-6-bromanyl-2-(carbamimidoylsulfanylmethyl)-1-methyl-indole-3-carboxylate;hydrobromide | octyl 5-acetyloxy-6-bromo-2-(carbamimidoylsulfanylmethyl)-1-methylindole-3-carboxylate;hydrobromide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H30BrN3O4S |
|---|
| Mol. Mass. | 512.46 |
|---|
| SMILES | CCCCCCCCOC(=O)c1c(CSC(N)=N)n(C)c2cc(Br)c(OC(C)=O)cc12 |
|---|
| Structure | 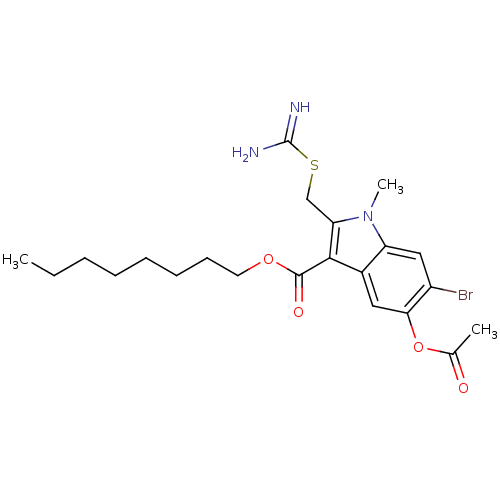
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yang, K; Nong, K; Gu, Q; Dong, J; Wang, J Discovery of N-hydroxy-3-alkoxybenzamides as direct acid sphingomyelinase inhibitors using a ligand-based pharmacophore model. Eur J Med Chem151:389-400 (2018) [PubMed] Article
Yang, K; Nong, K; Gu, Q; Dong, J; Wang, J Discovery of N-hydroxy-3-alkoxybenzamides as direct acid sphingomyelinase inhibitors using a ligand-based pharmacophore model. Eur J Med Chem151:389-400 (2018) [PubMed] Article