| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sialidase |
|---|
| Ligand | BDBM5231 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1751542 (CHEMBL4186302) |
|---|
| IC50 | 85±n/a nM |
|---|
| Citation |  Wang, Z; Cheng, LP; Zhang, XH; Pang, W; Li, L; Zhao, JL Design, synthesis and biological evaluation of novel oseltamivir derivatives as potent neuraminidase inhibitors. Bioorg Med Chem Lett27:5429-5435 (2017) [PubMed] Article Wang, Z; Cheng, LP; Zhang, XH; Pang, W; Li, L; Zhao, JL Design, synthesis and biological evaluation of novel oseltamivir derivatives as potent neuraminidase inhibitors. Bioorg Med Chem Lett27:5429-5435 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sialidase |
|---|
| Name: | Sialidase |
|---|
| Synonyms: | NANH_CLOPF | nanH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 42808.79 |
|---|
| Organism: | Clostridium perfringens |
|---|
| Description: | ChEMBL_991336 |
|---|
| Residue: | 382 |
|---|
| Sequence: | MCNKNNTFEKNLDISHKPEPLILFNKDNNIWNSKYFRIPNIQLLNDGTILTFSDIRYNGP
DDHAYIDIASARSTDFGKTWSYNIAMKNNRIDSTYSRVMDSTTVITNTGRIILIAGSWNT
NGNWAMTTSTRRSDWSVQMIYSDDNGLTWSNKIDLTKDSSKVKNQPSNTIGWLGGVGSGI
VMDDGTIVMPAQISLRENNENNYYSLIIYSKDNGETWTMGNKVPNSNTSENMVIELDGAL
IMSTRYDYSGYRAAYISHDLGTTWEIYEPLNGKILTGKGSGCQGSFIKATTSNGHRIGLI
SAPKNTKGEYIRDNIAVYMIDFDDLSKGVQEICIPYPEDGNKLGGGYSCLSFKNNHLGIV
YEANGNIEYQDLTPYYSLINKQ
|
|
|
|---|
| BDBM5231 |
|---|
| n/a |
|---|
| Name | BDBM5231 |
|---|
| Synonyms: | (3R,4R,5S)-5-amino-3-[butyl(ethyl)amino]-4-acetamidocyclohex-1-ene-1-carboxylic acid | C3-Aza Carbocyclic Analogue 3g |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H27N3O3 |
|---|
| Mol. Mass. | 297.3932 |
|---|
| SMILES | CCCCN(CC)[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:8| |
|---|
| Structure | 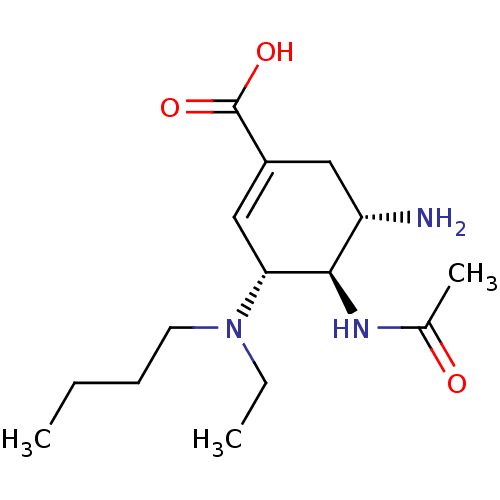
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wang, Z; Cheng, LP; Zhang, XH; Pang, W; Li, L; Zhao, JL Design, synthesis and biological evaluation of novel oseltamivir derivatives as potent neuraminidase inhibitors. Bioorg Med Chem Lett27:5429-5435 (2017) [PubMed] Article
Wang, Z; Cheng, LP; Zhang, XH; Pang, W; Li, L; Zhao, JL Design, synthesis and biological evaluation of novel oseltamivir derivatives as potent neuraminidase inhibitors. Bioorg Med Chem Lett27:5429-5435 (2017) [PubMed] Article