| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A5 |
|---|
| Ligand | BDBM50457696 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1760541 (CHEMBL4195549) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Santora, VJ; Almos, TA; Barido, R; Basinger, J; Bellows, CL; Bookser, BC; Breitenbucher, JG; Broadbent, NJ; Cabebe, C; Chai, CK; Chen, M; Chow, S; Chung, M; Crickard, L; Danks, AM; Freestone, GC; Gitnick, D; Gupta, V; Hoffmaster, C; Hudson, AR; Kaplan, AP; Kennedy, MR; Lee, D; Limberis, J; Ly, K; Mak, CC; Masatsugu, B; Morse, AC; Na, J; Neul, D; Nikpur, J; Peters, M; Petroski, RE; Renick, J; Sebring, K; Sevidal, S; Tabatabaei, A; Wen, J; Yan, Y; Yoder, ZW; Zook, D Design and Synthesis of Novel and Selective Glycine Transporter-1 (GlyT1) Inhibitors with Memory Enhancing Properties. J Med Chem61:6018-6033 (2018) [PubMed] Article Santora, VJ; Almos, TA; Barido, R; Basinger, J; Bellows, CL; Bookser, BC; Breitenbucher, JG; Broadbent, NJ; Cabebe, C; Chai, CK; Chen, M; Chow, S; Chung, M; Crickard, L; Danks, AM; Freestone, GC; Gitnick, D; Gupta, V; Hoffmaster, C; Hudson, AR; Kaplan, AP; Kennedy, MR; Lee, D; Limberis, J; Ly, K; Mak, CC; Masatsugu, B; Morse, AC; Na, J; Neul, D; Nikpur, J; Peters, M; Petroski, RE; Renick, J; Sebring, K; Sevidal, S; Tabatabaei, A; Wen, J; Yan, Y; Yoder, ZW; Zook, D Design and Synthesis of Novel and Selective Glycine Transporter-1 (GlyT1) Inhibitors with Memory Enhancing Properties. J Med Chem61:6018-6033 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A5 |
|---|
| Name: | Cytochrome P450 3A5 |
|---|
| Synonyms: | CP3A5_HUMAN | CYP3A5 | Cytochrome P450 3A5 | Cytochrome P450 3A5 (CYP3A5) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 57118.00 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P20815 |
|---|
| Residue: | 502 |
|---|
| Sequence: | MDLIPNLAVETWLLLAVSLVLLYLYGTRTHGLFKRLGIPGPTPLPLLGNVLSYRQGLWKF
DTECYKKYGKMWGTYEGQLPVLAITDPDVIRTVLVKECYSVFTNRRSLGPVGFMKSAISL
AEDEEWKRIRSLLSPTFTSGKLKEMFPIIAQYGDVLVRNLRREAEKGKPVTLKDIFGAYS
MDVITGTSFGVNIDSLNNPQDPFVESTKKFLKFGFLDPLFLSIILFPFLTPVFEALNVSL
FPKDTINFLSKSVNRMKKSRLNDKQKHRLDFLQLMIDSQNSKETESHKALSDLELAAQSI
IFIFAGYETTSSVLSFTLYELATHPDVQQKLQKEIDAVLPNKAPPTYDAVVQMEYLDMVV
NETLRLFPVAIRLERTCKKDVEINGVFIPKGSMVVIPTYALHHDPKYWTEPEEFRPERFS
KKKDSIDPYIYTPFGTGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLDTQG
LLQPEKPIVLKVDSRDGTLSGE
|
|
|
|---|
| BDBM50457696 |
|---|
| n/a |
|---|
| Name | BDBM50457696 |
|---|
| Synonyms: | CHEMBL4214486 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H18FN5O2 |
|---|
| Mol. Mass. | 391.3983 |
|---|
| SMILES | CC(C)Oc1ccc(cc1C(=O)N1Cc2cn(nc2C1)-c1cncc(F)c1)C#N |
|---|
| Structure | 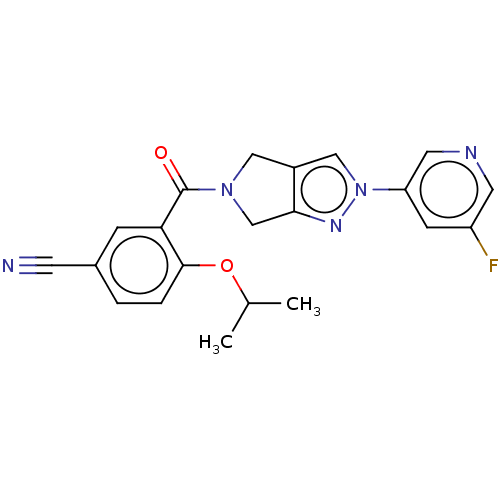
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Santora, VJ; Almos, TA; Barido, R; Basinger, J; Bellows, CL; Bookser, BC; Breitenbucher, JG; Broadbent, NJ; Cabebe, C; Chai, CK; Chen, M; Chow, S; Chung, M; Crickard, L; Danks, AM; Freestone, GC; Gitnick, D; Gupta, V; Hoffmaster, C; Hudson, AR; Kaplan, AP; Kennedy, MR; Lee, D; Limberis, J; Ly, K; Mak, CC; Masatsugu, B; Morse, AC; Na, J; Neul, D; Nikpur, J; Peters, M; Petroski, RE; Renick, J; Sebring, K; Sevidal, S; Tabatabaei, A; Wen, J; Yan, Y; Yoder, ZW; Zook, D Design and Synthesis of Novel and Selective Glycine Transporter-1 (GlyT1) Inhibitors with Memory Enhancing Properties. J Med Chem61:6018-6033 (2018) [PubMed] Article
Santora, VJ; Almos, TA; Barido, R; Basinger, J; Bellows, CL; Bookser, BC; Breitenbucher, JG; Broadbent, NJ; Cabebe, C; Chai, CK; Chen, M; Chow, S; Chung, M; Crickard, L; Danks, AM; Freestone, GC; Gitnick, D; Gupta, V; Hoffmaster, C; Hudson, AR; Kaplan, AP; Kennedy, MR; Lee, D; Limberis, J; Ly, K; Mak, CC; Masatsugu, B; Morse, AC; Na, J; Neul, D; Nikpur, J; Peters, M; Petroski, RE; Renick, J; Sebring, K; Sevidal, S; Tabatabaei, A; Wen, J; Yan, Y; Yoder, ZW; Zook, D Design and Synthesis of Novel and Selective Glycine Transporter-1 (GlyT1) Inhibitors with Memory Enhancing Properties. J Med Chem61:6018-6033 (2018) [PubMed] Article