| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50472848 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_47661 (CHEMBL657373) |
|---|
| Ki | 112±n/a nM |
|---|
| Citation |  Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem43:3596-613 (2000) [PubMed] Article Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem43:3596-613 (2000) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49676.37 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholecystokinin central 0 RAT::P30551 |
|---|
| Residue: | 444 |
|---|
| Sequence: | MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQI
LLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLK
DFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAAT
WCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVM
VVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQL
SSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAE
KHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEE
DGRTIRALLSRYSYSHMSTSAPPP
|
|
|
|---|
| BDBM50472848 |
|---|
| n/a |
|---|
| Name | BDBM50472848 |
|---|
| Synonyms: | CHEMBL420728 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H30N4O3 |
|---|
| Mol. Mass. | 470.5628 |
|---|
| SMILES | CC(C)(C)CCN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O |
|---|
| Structure | 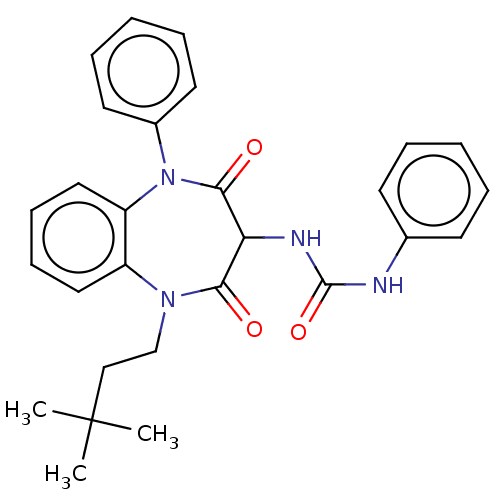
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem43:3596-613 (2000) [PubMed] Article
Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem43:3596-613 (2000) [PubMed] Article