

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419334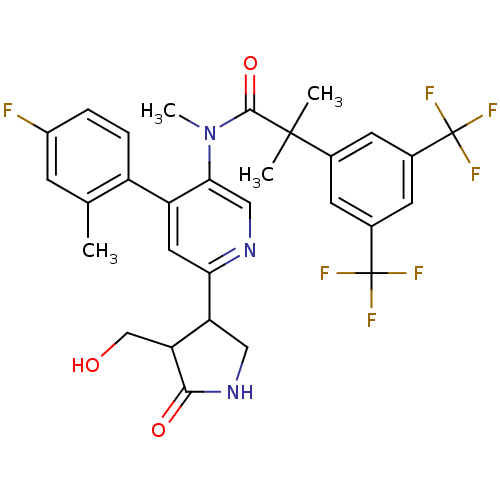 (CHEMBL1911965 | CHEMBL1911967) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419337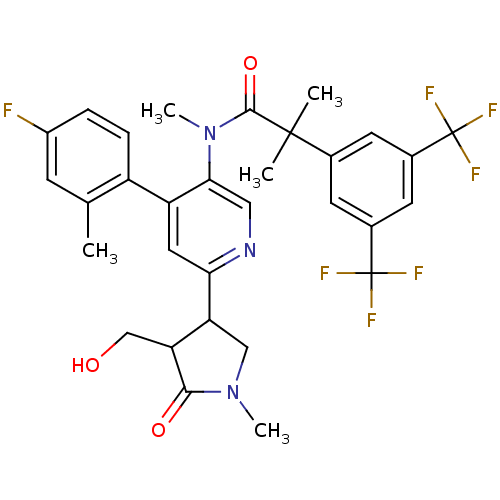 (CHEMBL1911968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419332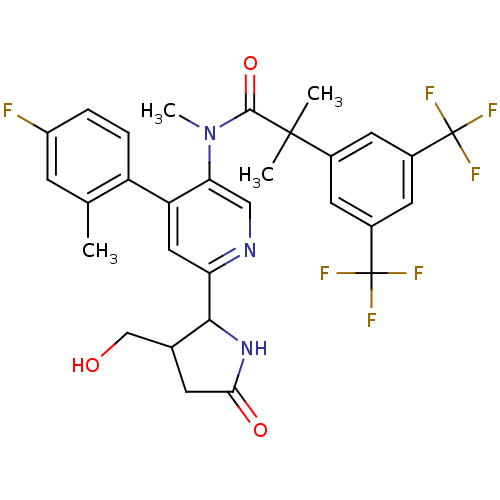 (CHEMBL1911964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419333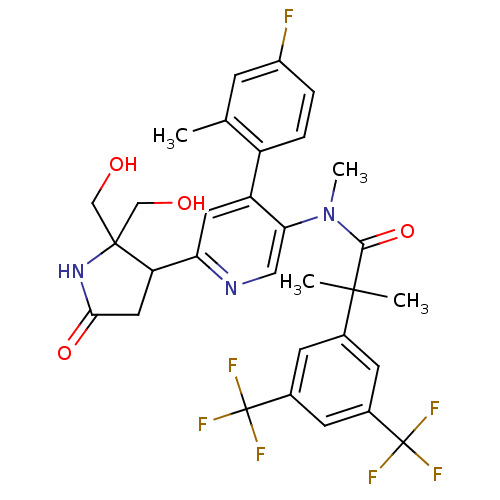 (CHEMBL1911970) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus (rat)) | BDBM50308250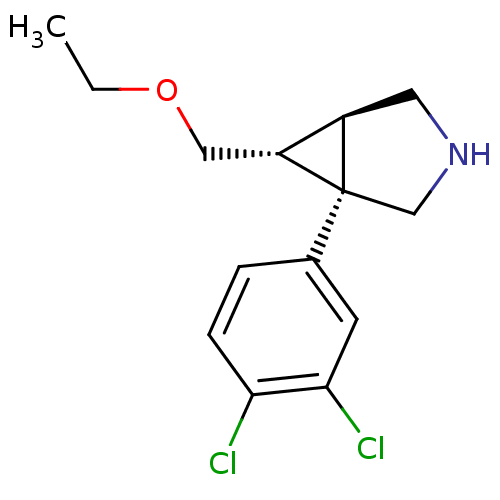 ((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...) | Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]nisoxetine from rat hippocampus NET by filtration binding assay | J Med Chem 53: 2534-51 (2010) Article DOI: 10.1021/jm901818u BindingDB Entry DOI: 10.7270/Q2ST7PX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463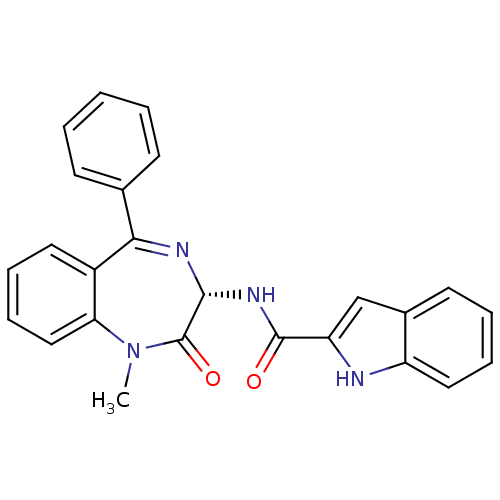 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM81962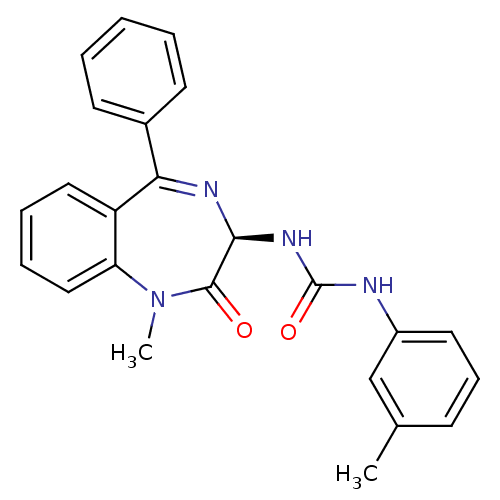 (S-L-365,260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50449787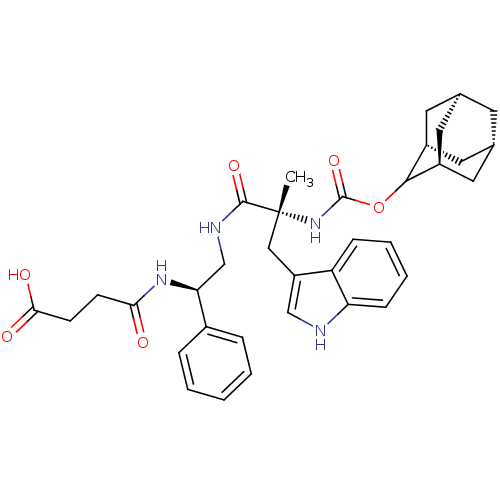 (CHEMBL2062154 | PD-134308) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419334 (CHEMBL1911965 | CHEMBL1911967) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472889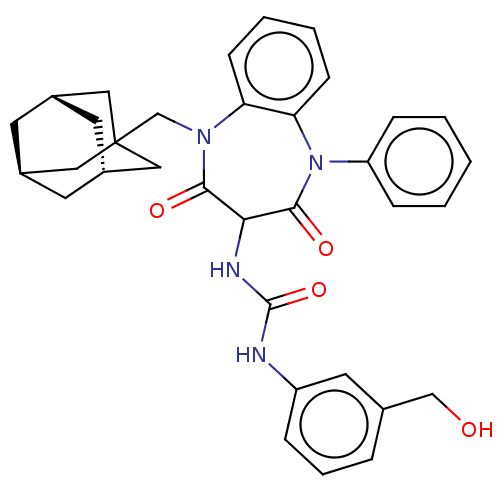 (CHEMBL415240) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472863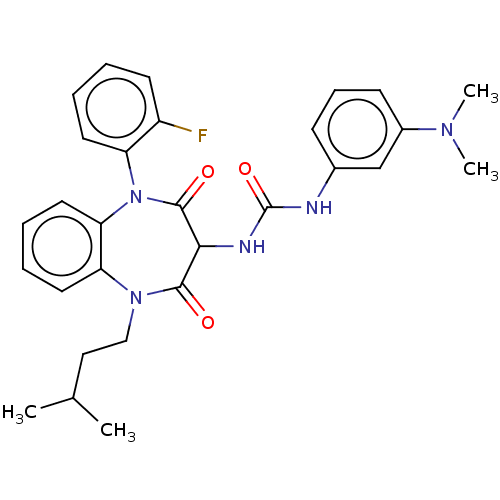 (CHEMBL333979) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472878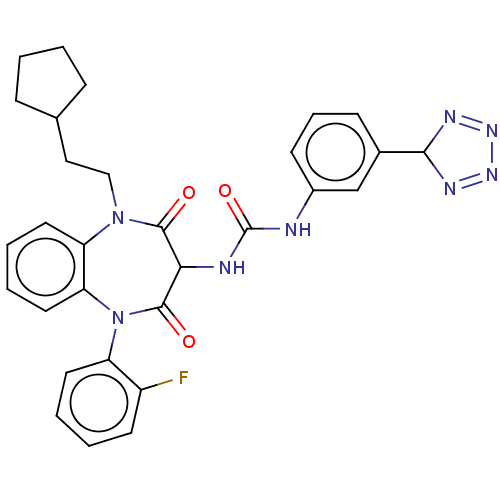 (CHEMBL331872) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419334 (CHEMBL1911965 | CHEMBL1911967) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419332 (CHEMBL1911964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419331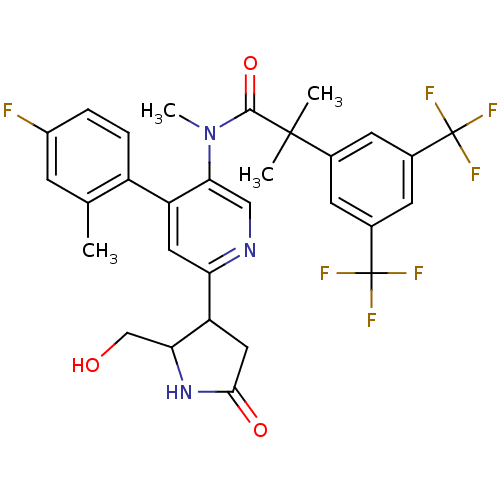 (CHEMBL1911969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419331 (CHEMBL1911969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472863 (CHEMBL333979) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472898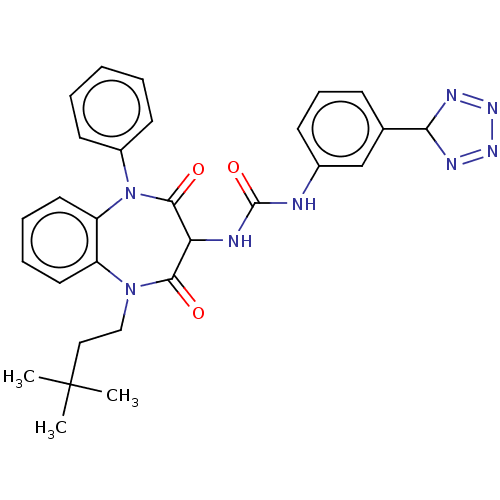 (CHEMBL332902) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472855 (CHEMBL331468) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472870 (CHEMBL331975) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419332 (CHEMBL1911964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002630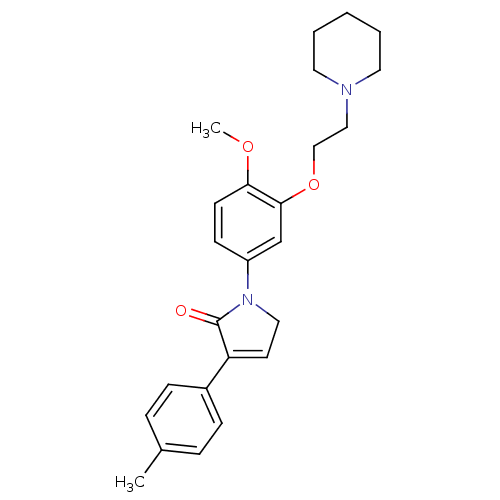 (CHEMBL377174) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472884 (CHEMBL121021) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50449787 (CHEMBL2062154 | PD-134308) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against human Cholecystokinin type B receptor | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472854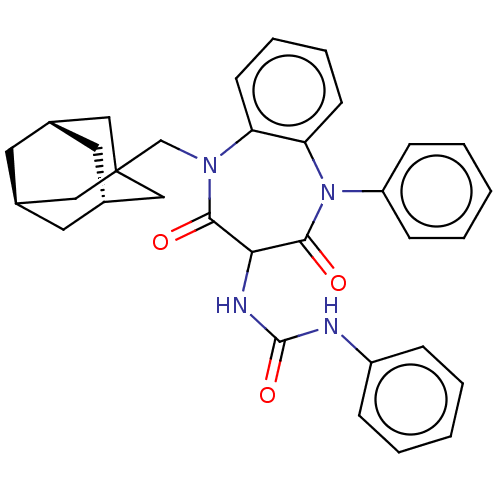 (CHEMBL330977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against human Cholecystokinin type B receptor | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50476485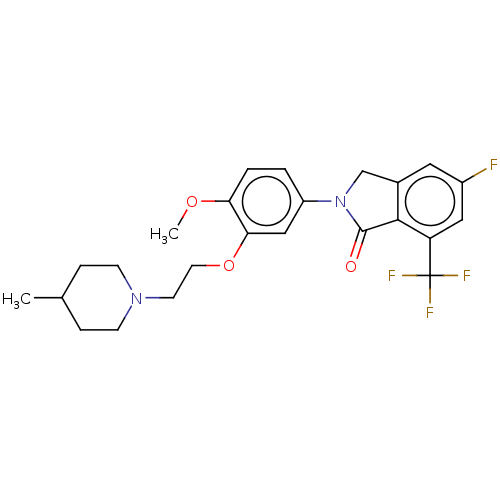 (CHEMBL234739) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 428-33 (2007) Article DOI: 10.1016/j.bmcl.2006.10.029 BindingDB Entry DOI: 10.7270/Q2TX3J4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472887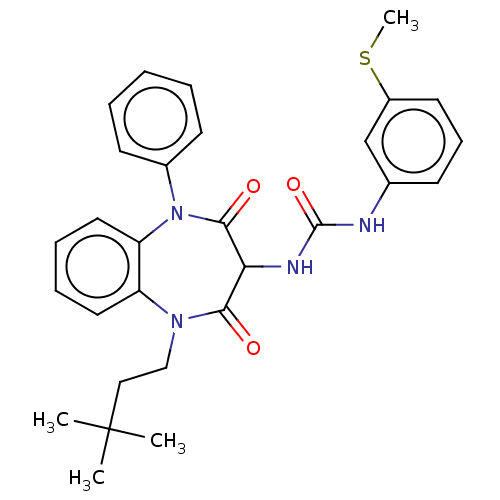 (CHEMBL330773) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419332 (CHEMBL1911964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NK1 receptor expressed in human U20S cells assessed as effect on substance P-induced intracellular calcium l... | Bioorg Med Chem Lett 21: 6899-904 (2011) Article DOI: 10.1016/j.bmcl.2011.07.116 BindingDB Entry DOI: 10.7270/Q24X592P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472864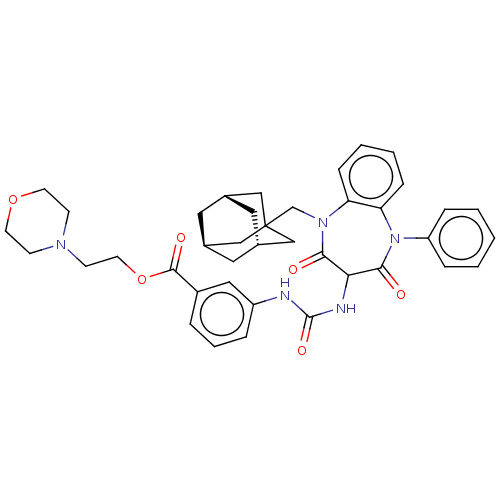 (CHEMBL402702) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472853 (CHEMBL331244) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472876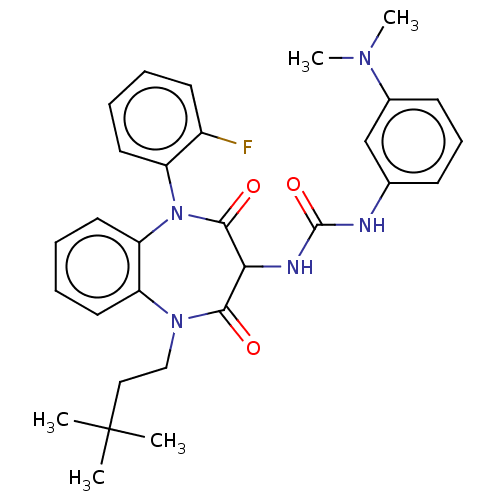 (CHEMBL332568) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472885 (CHEMBL118622) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472871 (CHEMBL118954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472886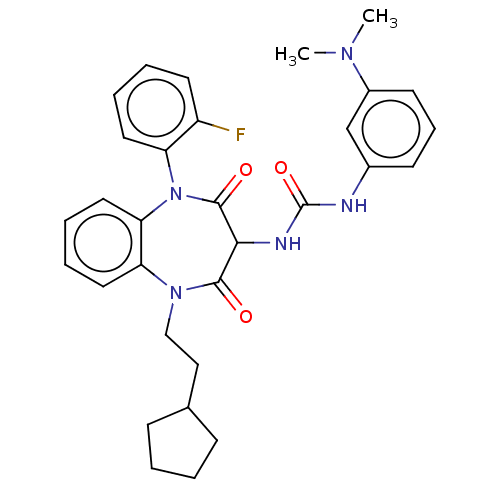 (CHEMBL421271) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472848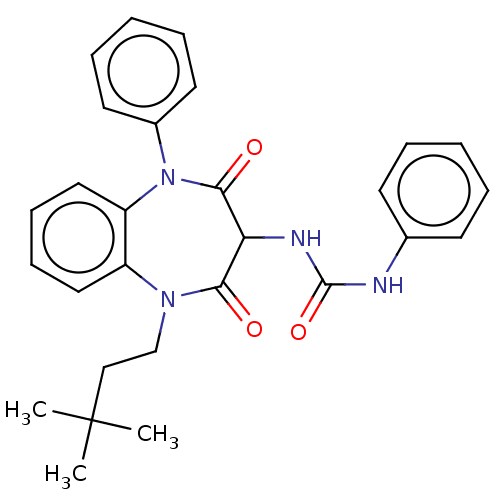 (CHEMBL420728) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472851 (CHEMBL331763) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472854 (CHEMBL330977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type B receptor of guinea pig | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472895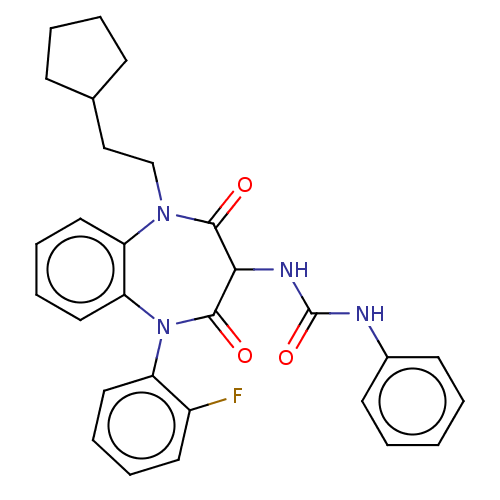 (CHEMBL120296) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472882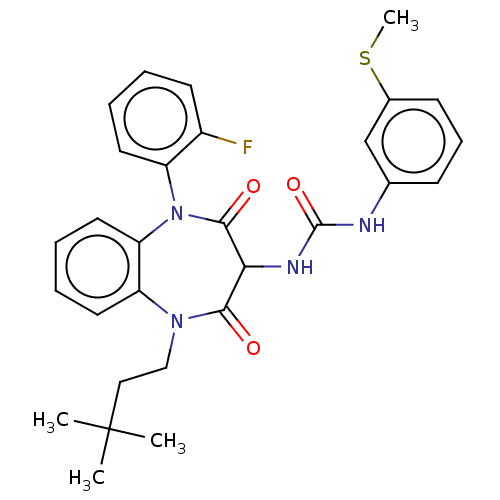 (CHEMBL332403) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472847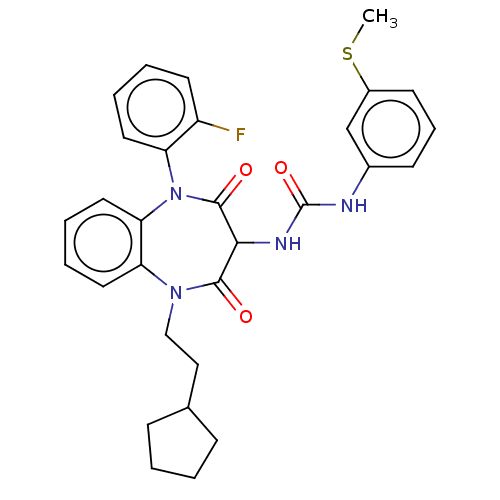 (CHEMBL121331) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002616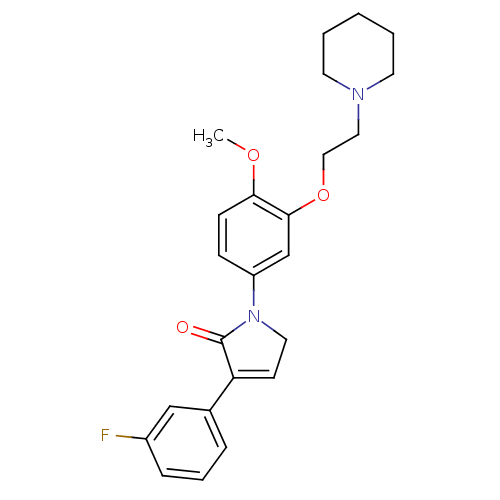 (CHEMBL213839) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002642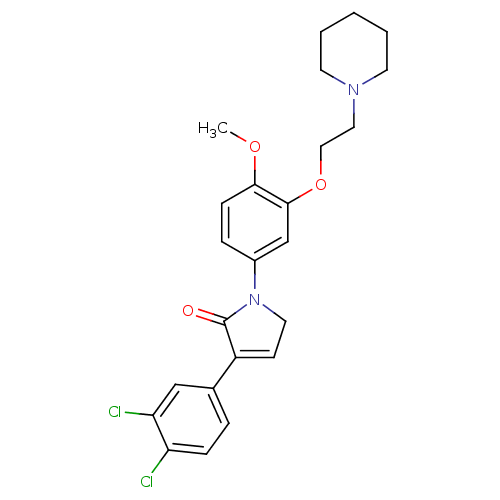 (CHEMBL213987) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50411716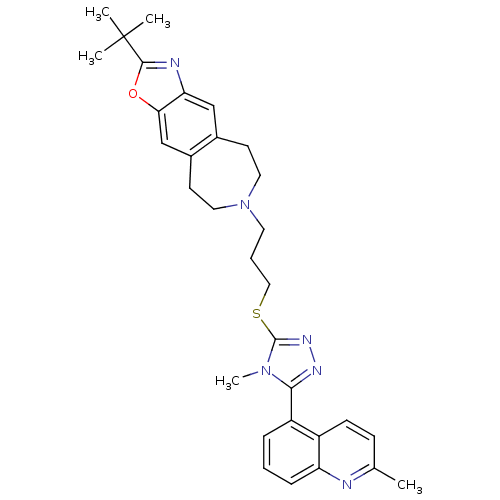 (CHEMBL429132) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor by cell based GTPgammaS binding assay | Bioorg Med Chem Lett 18: 901-7 (2008) Article DOI: 10.1016/j.bmcl.2007.12.066 BindingDB Entry DOI: 10.7270/Q2MK6F32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50476478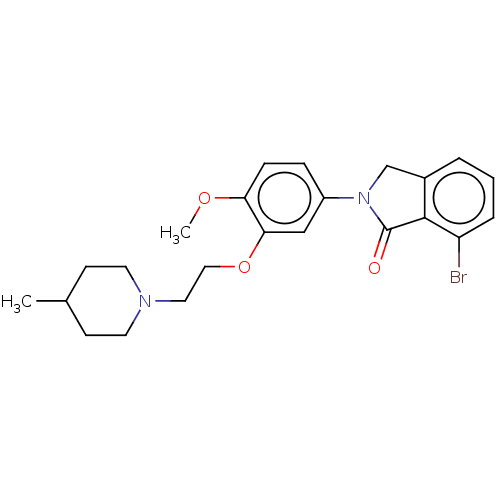 (CHEMBL234109) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 428-33 (2007) Article DOI: 10.1016/j.bmcl.2006.10.029 BindingDB Entry DOI: 10.7270/Q2TX3J4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472857 (CHEMBL121028) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (MOUSE) | BDBM50308250 ((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from mouse cortex SERT by filtration binding assay | J Med Chem 53: 2534-51 (2010) Article DOI: 10.1021/jm901818u BindingDB Entry DOI: 10.7270/Q2ST7PX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472854 (CHEMBL330977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472865 (CHEMBL331971) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472893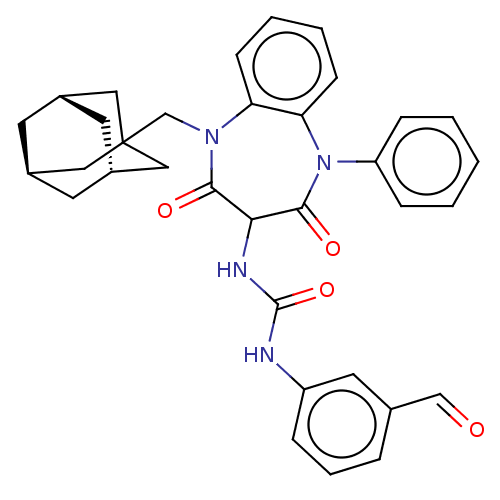 (CHEMBL333517) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472873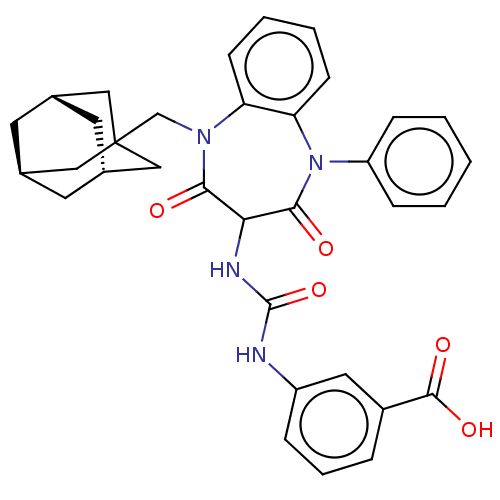 (CHEMBL120363) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1216 total ) | Next | Last >> |