| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Gastrin/cholecystokinin type B receptor |
|---|
| Ligand | BDBM50472854 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_48758 (CHEMBL666784) |
|---|
| Ki | 2.8±n/a nM |
|---|
| Citation |  Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem43:3596-613 (2000) [PubMed] Article Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem43:3596-613 (2000) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Gastrin/cholecystokinin type B receptor |
|---|
| Name: | Gastrin/cholecystokinin type B receptor |
|---|
| Synonyms: | Cckbr | Cholecystokinin A | Cholecystokinin B receptor | Cholecystokinin receptor | GASR_RAT | Gastrin/cholecystokinin type B receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 48980.43 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholecystokinin A CCKBR RAT::P30553 |
|---|
| Residue: | 452 |
|---|
| Sequence: | MELLKLNRSVQGPGPGSGSSLCRPGVSLLNSSSAGNLSCDPPRIRGTGTRELEMAIRITL
YAVIFLMSVGGNVLIIVVLGLSRRLRTVTNAFLLSLAVSDLLLAVACMPFTLLPNLMGTF
IFGTVICKAISYLMGVSVSVSTLNLVAIALERYSAICRPLQARVWQTRSHAARVILATWL
LSGLLMVPYPVYTMVQPVGPRVLQCMHRWPSARVQQTWSVLLLLLLFFIPGVVIAVAYGL
ISRELYLGLHFDGENDSETQSRARNQGGLPGGAAPGPVHQNGGCRPVTSVAGEDSDGCCV
QLPRSRLEMTTLTTPTPGPVPGPRPNQAKLLAKKRVVRMLLVIVLLFFLCWLPVYSVNTW
RAFDGPGAQRALSGAPISFIHLLSYVSACVNPLVYCFMHRRFRQACLDTCARCCPRPPRA
RPQPLPDEDPPTPSIASLSRLSYTTISTLGPG
|
|
|
|---|
| BDBM50472854 |
|---|
| n/a |
|---|
| Name | BDBM50472854 |
|---|
| Synonyms: | CHEMBL330977 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H34N4O3 |
|---|
| Mol. Mass. | 534.6481 |
|---|
| SMILES | [H][C@]12C[C@]3([H])C[C@]([H])(C1)CC(CN1c4ccccc4N(c4ccccc4)C(=O)C(NC(=O)Nc4ccccc4)C1=O)(C2)C3 |TLB:8:1:42:6.9.5,8:6:42:1.41.2,THB:2:3:9:1.41.8,2:1:9:3.42.5| |
|---|
| Structure | 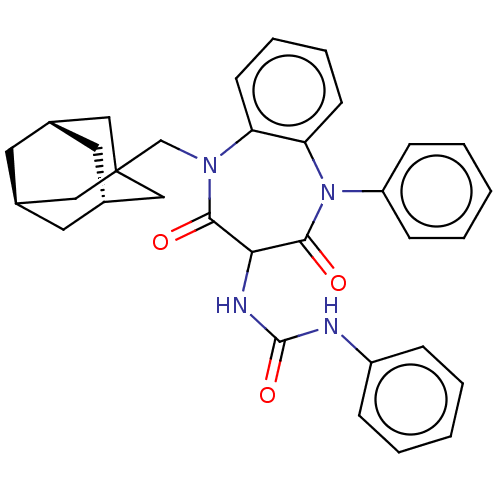
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem43:3596-613 (2000) [PubMed] Article
Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem43:3596-613 (2000) [PubMed] Article