| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase |
|---|
| Ligand | BDBM50484284 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_768443 (CHEMBL1832162) |
|---|
| IC50 | 13900±n/a nM |
|---|
| Citation |  Barral, K; Weck, C; Payrot, N; Roux, L; Durafour, C; Zoulim, F; Neyts, J; Balzarini, J; Canard, B; Priet, S; Alvarez, K Acyclic nucleoside thiophosphonates as potent inhibitors of HIV and HBV replication. Eur J Med Chem46:4281-8 (2011) [PubMed] Article Barral, K; Weck, C; Payrot, N; Roux, L; Durafour, C; Zoulim, F; Neyts, J; Balzarini, J; Canard, B; Priet, S; Alvarez, K Acyclic nucleoside thiophosphonates as potent inhibitors of HIV and HBV replication. Eur J Med Chem46:4281-8 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase |
|---|
| Name: | Reverse transcriptase |
|---|
| Synonyms: | n/a |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 29598.37 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | Q9WKE8 |
|---|
| Residue: | 254 |
|---|
| Sequence: | PISPITVPVKLKPGMDGPKVKQWPLTEEKIKALTEICTEMEKEGKIEKIGPENPYNTPVF
AIKKKDSTKWRKVVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPLD
KDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVIY
QYMDDLYVGSDLEIEQHRAKIEELRQHLLRWGFTTPDKKHQKEPPFLWMGYELHPDKWTV
QPIVLPEKDSWTVN
|
|
|
|---|
| BDBM50484284 |
|---|
| n/a |
|---|
| Name | BDBM50484284 |
|---|
| Synonyms: | CHEMBL1830072 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C9H12N5Na4O9P3S |
|---|
| Mol. Mass. | 551.165 |
|---|
| SMILES | [Na;v0+].[Na;v0+].[Na;v0+].[Na;v0+].[#6]-[#6@H](-[#6]-n1cnc2c(-[#7])ncnc12)-[#8]-[#6]P([#16-])(=O)[#8]P([#8-])(=O)[#8]P([#8-])([#8-])=O |r| |
|---|
| Structure | 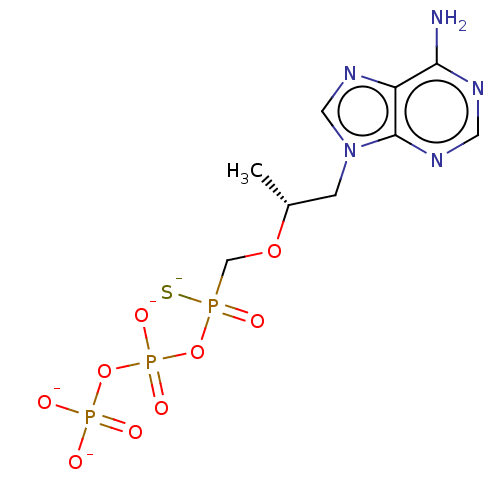
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Barral, K; Weck, C; Payrot, N; Roux, L; Durafour, C; Zoulim, F; Neyts, J; Balzarini, J; Canard, B; Priet, S; Alvarez, K Acyclic nucleoside thiophosphonates as potent inhibitors of HIV and HBV replication. Eur J Med Chem46:4281-8 (2011) [PubMed] Article
Barral, K; Weck, C; Payrot, N; Roux, L; Durafour, C; Zoulim, F; Neyts, J; Balzarini, J; Canard, B; Priet, S; Alvarez, K Acyclic nucleoside thiophosphonates as potent inhibitors of HIV and HBV replication. Eur J Med Chem46:4281-8 (2011) [PubMed] Article