| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Endothelin receptor type B |
|---|
| Ligand | BDBM50041617 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_63849 (CHEMBL673233) |
|---|
| Ki | 14.7±n/a nM |
|---|
| Citation |  Elliott, JD; Lago, MA; Cousins, RD; Gao, A; Leber, JD; Erhard, KF; Nambi, P; Elshourbagy, NA; Kumar, C; Lee, JA 1,3-Diarylindan-2-carboxylic acids, potent and selective non-peptide endothelin receptor antagonists. J Med Chem37:1553-7 (1994) [PubMed] Elliott, JD; Lago, MA; Cousins, RD; Gao, A; Leber, JD; Erhard, KF; Nambi, P; Elshourbagy, NA; Kumar, C; Lee, JA 1,3-Diarylindan-2-carboxylic acids, potent and selective non-peptide endothelin receptor antagonists. J Med Chem37:1553-7 (1994) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Endothelin receptor type B |
|---|
| Name: | Endothelin receptor type B |
|---|
| Synonyms: | EDNRB | EDNRB_HUMAN | ENDOTHELIN B | ET-B | ETRB | Endothelin receptor ET-B | Endothelin receptor non-selective type | Endothelin receptor, ET-A/ET-B |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49664.00 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ENDOTHELIN B EDNRB HUMAN::P24530 |
|---|
| Residue: | 442 |
|---|
| Sequence: | MQPPPSLCGRALVALVLACGLSRIWGEERGFPPDRATPLLQTAEIMTPPTKTLWPKGSNA
SLARSLAPAEVPKGDRTAGSPPRTISPPPCQGPIEIKETFKYINTVVSCLVFVLGIIGNS
TLLRIIYKNKCMRNGPNILIASLALGDLLHIVIDIPINVYKLLAEDWPFGAEMCKLVPFI
QKASVGITVLSLCALSIDRYRAVASWSRIKGIGVPKWTAVEIVLIWVVSVVLAVPEAIGF
DIITMDYKGSYLRICLLHPVQKTAFMQFYKTAKDWWLFSFYFCLPLAITAFFYTLMTCEM
LRKKSGMQIALNDHLKQRREVAKTVFCLVLVFALCWLPLHLSRILKLTLYNQNDPNRCEL
LSFLLVLDYIGINMASLNSCINPIALYLVSKRFKNCFKSCLCCWCQSFEEKQSLEEKQSC
LKFKANDHGYDNFRSSNKYSSS
|
|
|
|---|
| BDBM50041617 |
|---|
| n/a |
|---|
| Name | BDBM50041617 |
|---|
| Synonyms: | (1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymethoxy-4-methoxy-phenyl)-5-propoxy-indan-2-carboxylic acid | 1-benzo[d][1,3]dioxol-5-ylmethyl-3-(2-carboxymethoxy-4-methoxyphenyl)-5-propoxy-(1R,3S)-2,3-dihydro-1H-2-indenecarboxylic acid | CHEMBL8823 | SB 209670 | SB-209670 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H28O9 |
|---|
| Mol. Mass. | 520.5272 |
|---|
| SMILES | CCCOc1ccc2[C@@H]([C@H]([C@@H](c2c1)c1ccc(OC)cc1OCC(O)=O)C(O)=O)c1ccc2OCOc2c1 |
|---|
| Structure | 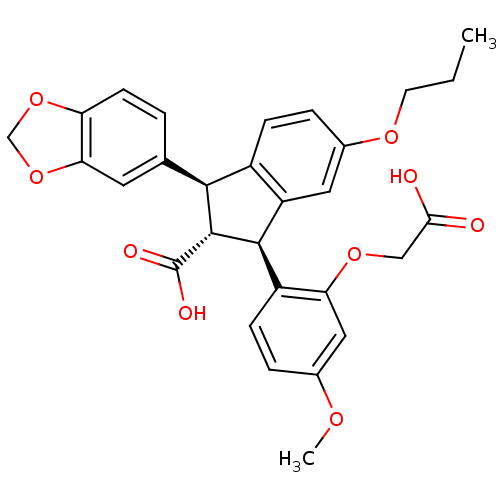
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Elliott, JD; Lago, MA; Cousins, RD; Gao, A; Leber, JD; Erhard, KF; Nambi, P; Elshourbagy, NA; Kumar, C; Lee, JA 1,3-Diarylindan-2-carboxylic acids, potent and selective non-peptide endothelin receptor antagonists. J Med Chem37:1553-7 (1994) [PubMed]
Elliott, JD; Lago, MA; Cousins, RD; Gao, A; Leber, JD; Erhard, KF; Nambi, P; Elshourbagy, NA; Kumar, C; Lee, JA 1,3-Diarylindan-2-carboxylic acids, potent and selective non-peptide endothelin receptor antagonists. J Med Chem37:1553-7 (1994) [PubMed]