| Reaction Details |
|---|
 | Report a problem with these data |
| Target | DNA (cytosine-5)-methyltransferase 1 |
|---|
| Ligand | BDBM50495477 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1287366 (CHEMBL3111348) |
|---|
| IC50 | 40000±n/a nM |
|---|
| Citation |  Asgatay, S; Champion, C; Marloie, G; Drujon, T; Senamaud-Beaufort, C; Ceccaldi, A; Erdmann, A; Rajavelu, A; Schambel, P; Jeltsch, A; Lequin, O; Karoyan, P; Arimondo, PB; Guianvarc'h, D Synthesis and evaluation of analogues of N-phthaloyl-l-tryptophan (RG108) as inhibitors of DNA methyltransferase 1. J Med Chem57:421-34 (2014) [PubMed] Article Asgatay, S; Champion, C; Marloie, G; Drujon, T; Senamaud-Beaufort, C; Ceccaldi, A; Erdmann, A; Rajavelu, A; Schambel, P; Jeltsch, A; Lequin, O; Karoyan, P; Arimondo, PB; Guianvarc'h, D Synthesis and evaluation of analogues of N-phthaloyl-l-tryptophan (RG108) as inhibitors of DNA methyltransferase 1. J Med Chem57:421-34 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| DNA (cytosine-5)-methyltransferase 1 |
|---|
| Name: | DNA (cytosine-5)-methyltransferase 1 |
|---|
| Synonyms: | AIM | CXXC-type zinc finger protein 9 | CXXC9 | DNA (cytosine-5)-methyltransferase 1 (DNMT1) | DNA (cytosine-5)-methyltransferase 1 isoform b | DNA MTase HsaI | DNA methyltransferase HsaI | DNMT | DNMT1 | DNMT1_HUMAN | M.HsaI | MCMT |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 183184.85 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P26358 |
|---|
| Residue: | 1616 |
|---|
| Sequence: | MPARTAPARVPTLAVPAISLPDDVRRRLKDLERDSLTEKECVKEKLNLLHEFLQTEIKNQ
LCDLETKLRKEELSEEGYLAKVKSLLNKDLSLENGAHAYNREVNGRLENGNQARSEARRV
GMADANSPPKPLSKPRTPRRSKSDGEAKPEPSPSPRITRKSTRQTTITSHFAKGPAKRKP
QEESERAKSDESIKEEDKDQDEKRRRVTSRERVARPLPAEEPERAKSGTRTEKEEERDEK
EEKRLRSQTKEPTPKQKLKEEPDREARAGVQADEDEDGDEKDEKKHRSQPKDLAAKRRPE
EKEPEKVNPQISDEKDEDEKEEKRRKTTPKEPTEKKMARAKTVMNSKTHPPKCIQCGQYL
DDPDLKYGQHPPDAVDEPQMLTNEKLSIFDANESGFESYEALPQHKLTCFSVYCKHGHLC
PIDTGLIEKNIELFFSGSAKPIYDDDPSLEGGVNGKNLGPINEWWITGFDGGEKALIGFS
TSFAEYILMDPSPEYAPIFGLMQEKIYISKIVVEFLQSNSDSTYEDLINKIETTVPPSGL
NLNRFTEDSLLRHAQFVVEQVESYDEAGDSDEQPIFLTPCMRDLIKLAGVTLGQRRAQAR
RQTIRHSTREKDRGPTKATTTKLVYQIFDTFFAEQIEKDDREDKENAFKRRRCGVCEVCQ
QPECGKCKACKDMVKFGGSGRSKQACQERRCPNMAMKEADDDEEVDDNIPEMPSPKKMHQ
GKKKKQNKNRISWVGEAVKTDGKKSYYKKVCIDAETLEVGDCVSVIPDDSSKPLYLARVT
ALWEDSSNGQMFHAHWFCAGTDTVLGATSDPLELFLVDECEDMQLSYIHSKVKVIYKAPS
ENWAMEGGMDPESLLEGDDGKTYFYQLWYDQDYARFESPPKTQPTEDNKFKFCVSCARLA
EMRQKEIPRVLEQLEDLDSRVLYYSATKNGILYRVGDGVYLPPEAFTFNIKLSSPVKRPR
KEPVDEDLYPEHYRKYSDYIKGSNLDAPEPYRIGRIKEIFCPKKSNGRPNETDIKIRVNK
FYRPENTHKSTPASYHADINLLYWSDEEAVVDFKAVQGRCTVEYGEDLPECVQVYSMGGP
NRFYFLEAYNAKSKSFEDPPNHARSPGNKGKGKGKGKGKPKSQACEPSEPEIEIKLPKLR
TLDVFSGCGGLSEGFHQAGISDTLWAIEMWDPAAQAFRLNNPGSTVFTEDCNILLKLVMA
GETTNSRGQRLPQKGDVEMLCGGPPCQGFSGMNRFNSRTYSKFKNSLVVSFLSYCDYYRP
RFFLLENVRNFVSFKRSMVLKLTLRCLVRMGYQCTFGVLQAGQYGVAQTRRRAIILAAAP
GEKLPLFPEPLHVFAPRACQLSVVVDDKKFVSNITRLSSGPFRTITVRDTMSDLPEVRNG
ASALEISYNGEPQSWFQRQLRGAQYQPILRDHICKDMSALVAARMRHIPLAPGSDWRDLP
NIEVRLSDGTMARKLRYTHHDRKNGRSSSGALRGVCSCVEAGKACDPAARQFNTLIPWCL
PHTGNRHNHWAGLYGRLEWDGFFSTTVTNPEPMGKQGRVLHPEQHRVVSVRECARSQGFP
DTYRLFGNILDKHRQVGNAVPPPLAKAIGLEIKLCMLAKARESASAKIKEEEAAKD
|
|
|
|---|
| BDBM50495477 |
|---|
| n/a |
|---|
| Name | BDBM50495477 |
|---|
| Synonyms: | CHEMBL3109085 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H14N4O6S |
|---|
| Mol. Mass. | 402.381 |
|---|
| SMILES | OC(=O)[C@H](Cc1c[nH]c2ccccc12)NSc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O |r| |
|---|
| Structure | 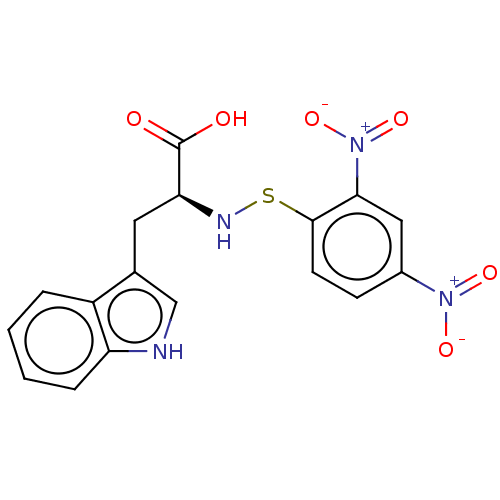
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Asgatay, S; Champion, C; Marloie, G; Drujon, T; Senamaud-Beaufort, C; Ceccaldi, A; Erdmann, A; Rajavelu, A; Schambel, P; Jeltsch, A; Lequin, O; Karoyan, P; Arimondo, PB; Guianvarc'h, D Synthesis and evaluation of analogues of N-phthaloyl-l-tryptophan (RG108) as inhibitors of DNA methyltransferase 1. J Med Chem57:421-34 (2014) [PubMed] Article
Asgatay, S; Champion, C; Marloie, G; Drujon, T; Senamaud-Beaufort, C; Ceccaldi, A; Erdmann, A; Rajavelu, A; Schambel, P; Jeltsch, A; Lequin, O; Karoyan, P; Arimondo, PB; Guianvarc'h, D Synthesis and evaluation of analogues of N-phthaloyl-l-tryptophan (RG108) as inhibitors of DNA methyltransferase 1. J Med Chem57:421-34 (2014) [PubMed] Article