| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Transcription initiation factor TFIID subunit 1 |
|---|
| Ligand | BDBM26514 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1809384 (CHEMBL4308743) |
|---|
| IC50 | 150±n/a nM |
|---|
| Citation |  Remillard, D; Buckley, DL; Seo, HS; Ferguson, FM; Dhe-Paganon, S; Bradner, JE; Gray, NS Dual Inhibition of TAF1 and BET Bromodomains from the BI-2536 Kinase Inhibitor Scaffold. ACS Med Chem Lett10:1443-1449 (2019) [PubMed] Article Remillard, D; Buckley, DL; Seo, HS; Ferguson, FM; Dhe-Paganon, S; Bradner, JE; Gray, NS Dual Inhibition of TAF1 and BET Bromodomains from the BI-2536 Kinase Inhibitor Scaffold. ACS Med Chem Lett10:1443-1449 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Transcription initiation factor TFIID subunit 1 |
|---|
| Name: | Transcription initiation factor TFIID subunit 1 |
|---|
| Synonyms: | BA2R | BA2R | CCG1 | CCGS | Cell cycle gene 1 protein | TAF(II)250 | TAF1 | TAF1_HUMAN | TAF2A | TAFII-250 | TAFII250 | TBP-associated factor 250 kDa | Transcription initiation factor TFIID 250 kDa subunit | Transcription initiation factor TFIID subunit 1 | p250 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 212598.75 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_108248 |
|---|
| Residue: | 1872 |
|---|
| Sequence: | MGPGCDLLLRTAATITAAAIMSDTDSDEDSAGGGPFSLAGFLFGNINGAGQLEGESVLDD
ECKKHLAGLGALGLGSLITELTANEELTGTDGALVNDEGWVRSTEDAVDYSDINEVAEDE
SRRYQQTMGSLQPLCHSDYDEDDYDADCEDIDCKLMPPPPPPPGPMKKDKDQDSITGEKV
DFSSSSDSESEMGPQEATQAESEDGKLTLPLAGIMQHDATKLLPSVTELFPEFRPGKVLR
FLRLFGPGKNVPSVWRSARRKRKKKHRELIQEEQIQEVECSVESEVSQKSLWNYDYAPPP
PPEQCLSDDEITMMAPVESKFSQSTGDIDKVTDTKPRVAEWRYGPARLWYDMLGVPEDGS
GFDYGFKLRKTEHEPVIKSRMIEEFRKLEENNGTDLLADENFLMVTQLHWEDDIIWDGED
VKHKGTKPQRASLAGWLPSSMTRNAMAYNVQQGFAATLDDDKPWYSIFPIDNEDLVYGRW
EDNIIWDAQAMPRLLEPPVLTLDPNDENLILEIPDEKEEATSNSPSKESKKESSLKKSRI
LLGKTGVIKEEPQQNMSQPEVKDPWNLSNDEYYYPKQQGLRGTFGGNIIQHSIPAVELRQ
PFFPTHMGPIKLRQFHRPPLKKYSFGALSQPGPHSVQPLLKHIKKKAKMREQERQASGGG
EMFFMRTPQDLTGKDGDLILAEYSEENGPLMMQVGMATKIKNYYKRKPGKDPGAPDCKYG
ETVYCHTSPFLGSLHPGQLLQAFENNLFRAPIYLHKMPETDFLIIRTRQGYYIRELVDIF
VVGQQCPLFEVPGPNSKRANTHIRDFLQVFIYRLFWKSKDRPRRIRMEDIKKAFPSHSES
SIRKRLKLCADFKRTGMDSNWWVLKSDFRLPTEEEIRAMVSPEQCCAYYSMIAAEQRLKD
AGYGEKSFFAPEEENEEDFQMKIDDEVRTAPWNTTRAFIAAMKGKCLLEVTGVADPTGCG
EGFSYVKIPNKPTQQKDDKEPQPVKKTVTGTDADLRRLSLKNAKQLLRKFGVPEEEIKKL
SRWEVIDVVRTMSTEQARSGEGPMSKFARGSRFSVAEHQERYKEECQRIFDLQNKVLSST
EVLSTDTDSSSAEDSDFEEMGKNIENMLQNKKTSSQLSREREEQERKELQRMLLAAGSAA
SGNNHRDDDTASVTSLNSSATGRCLKIYRTFRDEEGKEYVRCETVRKPAVIDAYVRIRTT
KDEEFIRKFALFDEQHREEMRKERRRIQEQLRRLKRNQEKEKLKGPPEKKPKKMKERPDL
KLKCGACGAIGHMRTNKFCPLYYQTNAPPSNPVAMTEEQEEELEKTVIHNDNEELIKVEG
TKIVLGKQLIESADEVRRKSLVLKFPKQQLPPKKKRRVGTTVHCDYLNRPHKSIHRRRTD
PMVTLSSILESIINDMRDLPNTYPFHTPVNAKVVKDYYKIITRPMDLQTLRENVRKRLYP
SREEFREHLELIVKNSATYNGPKHSLTQISQSMLDLCDEKLKEKEDKLARLEKAINPLLD
DDDQVAFSFILDNIVTQKMMAVPDSWPFHHPVNKKFVPDYYKVIVNPMDLETIRKNISKH
KYQSRESFLDDVNLILANSVKYNGPESQYTKTAQEIVNVCYQTLTEYDEHLTQLEKDICT
AKEAALEEAELESLDPMTPGPYTPQPPDLYDTNTSLSMSRDASVFQDESNMSVLDIPSAT
PEKQVTQEGEDGDGDLADEEEGTVQQPQASVLYEDLLMSEGEDDEEDAGSDEEGDNPFSA
IQLSESGSDSDVGSGGIRPKQPRMLQENTRMDMENEESMMSYEGDGGEASHGLEDSNISY
GSYEEPDPKSNTQDTSFSSIGGYEVSEEEEDEEEEEQRSGPSVLSQVHLSEDEEDSEDFH
SIAGDSDLDSDE
|
|
|
|---|
| BDBM26514 |
|---|
| n/a |
|---|
| Name | BDBM26514 |
|---|
| Synonyms: | 4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl]amino}-N-(1-methylpiperidin-4-yl)benzamide | BI 2536 analogue, 1b |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H37N7O2 |
|---|
| Mol. Mass. | 491.6284 |
|---|
| SMILES | CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r| |
|---|
| Structure | 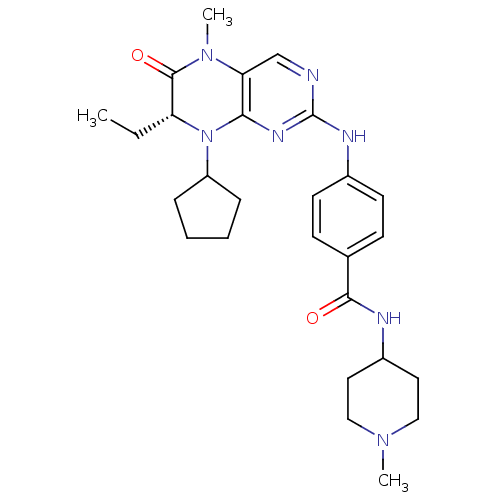
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Remillard, D; Buckley, DL; Seo, HS; Ferguson, FM; Dhe-Paganon, S; Bradner, JE; Gray, NS Dual Inhibition of TAF1 and BET Bromodomains from the BI-2536 Kinase Inhibitor Scaffold. ACS Med Chem Lett10:1443-1449 (2019) [PubMed] Article
Remillard, D; Buckley, DL; Seo, HS; Ferguson, FM; Dhe-Paganon, S; Bradner, JE; Gray, NS Dual Inhibition of TAF1 and BET Bromodomains from the BI-2536 Kinase Inhibitor Scaffold. ACS Med Chem Lett10:1443-1449 (2019) [PubMed] Article